Characterization of bortezomib-adapted I-45 mesothelioma cells
- PMID: 20482802
- PMCID: PMC2882347
- DOI: 10.1186/1476-4598-9-110
Characterization of bortezomib-adapted I-45 mesothelioma cells
Abstract
Background: Bortezomib, a proteasome-specific inhibitor, has emerged as a promising cancer therapeutic agent. However, development of resistance to bortezomib may pose a challenge to effective anticancer therapy. Therefore, characterization of cellular mechanisms involved in bortezomib resistance and development of effective strategies to overcome this resistance represent important steps in the advancement of bortezomib-mediated cancer therapy.
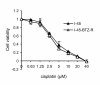
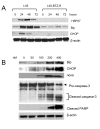
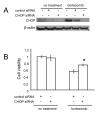
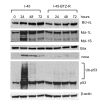
Results: The present study reports the development of I-45-BTZ-R, a bortezomib-resistant cell line, from the bortezomib-sensitive mesothelioma cell line I-45. I-45-BTZ-R cells showed no cross-resistance to the chemotherapeutic drugs cisplatin, 5-fluorouracil, and doxorubicin. Moreover, the bortezomib-adapted I-45-BTZ-R cells had decreased growth kinemics and did not over express proteasome subunit beta5 (PSMB5) as compared to parental I-45 cells. I-45-BTZ-R cells and parental I-45 cells showed similar inhibition of proteasome activity, but I-45-BTZ-R cells exhibited much less accumulation of ubiquitinated proteins following exposure to 40 nm bortezomib. Further studies revealed that relatively low doses of bortezomib did not induce an unfolded protein response (UPR) in the bortezomib-adapted cells, while higher doses induced UPR with concomitant cell death, as evidenced by higher expression of the mitochondrial chaperone protein Bip and the endoplasmic reticulum (ER) stress-related pro-apoptotic protein CHOP. In addition, bortezomib exposure did not induce the accumulation of the pro-apoptotic proteins p53, Mcl-1S, and noxa in the bortezomib-adapted cells.
Conclusion: These results suggest that UPR evasion, together with reduced pro-apoptotic gene induction, accounts for bortezomib resistance in the bortezomib-adapted mesothelioma cell line I-45-BTZ-R.
Figures


Similar articles
-
Perturbation of proteasome function by bortezomib leading to ER stress-induced apoptotic cell death in cholangiocarcinoma.J Cancer Res Clin Oncol. 2013 Sep;139(9):1551-62. doi: 10.1007/s00432-013-1473-6. Epub 2013 Jul 23. J Cancer Res Clin Oncol. 2013. PMID: 23877657 Free PMC article.
-
Bortezomib-resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress.Leukemia. 2010 Aug;24(8):1506-12. doi: 10.1038/leu.2010.137. Epub 2010 Jun 17. Leukemia. 2010. PMID: 20555361
-
Vorinostat eliminates multicellular resistance of mesothelioma 3D spheroids via restoration of Noxa expression.PLoS One. 2012;7(12):e52753. doi: 10.1371/journal.pone.0052753. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300762 Free PMC article.
-
Advances in the understanding of mechanisms and therapeutic use of bortezomib.Discov Med. 2011 Dec;12(67):471-80. Discov Med. 2011. PMID: 22204764 Free PMC article. Review.
-
Targeting endoplasmic reticulum signaling pathways in cancer.Acta Oncol. 2012 Sep;51(7):822-30. doi: 10.3109/0284186X.2012.689113. Epub 2012 Jun 11. Acta Oncol. 2012. PMID: 22686473 Review.
Cited by
-
The future of proteasome inhibitors in relapsed/refractory multiple myeloma.Oncology (Williston Park). 2011 Nov 15;25 Suppl 2(12 0 2):56-64. Oncology (Williston Park). 2011. PMID: 25188482 Free PMC article. Review.
-
Proteasome inhibitors - molecular basis and current perspectives in multiple myeloma.J Cell Mol Med. 2014 Jun;18(6):947-61. doi: 10.1111/jcmm.12279. Epub 2014 Apr 8. J Cell Mol Med. 2014. PMID: 24712303 Free PMC article. Review.
-
Endoplasmic reticulum stress and quality control in relation to cisplatin resistance in tumor cells.Front Pharmacol. 2024 Jun 14;15:1419468. doi: 10.3389/fphar.2024.1419468. eCollection 2024. Front Pharmacol. 2024. PMID: 38948460 Free PMC article. Review.
-
Repurposing host-based therapeutics to control coronavirus and influenza virus.Drug Discov Today. 2019 Mar;24(3):726-736. doi: 10.1016/j.drudis.2019.01.018. Epub 2019 Jan 31. Drug Discov Today. 2019. PMID: 30711575 Free PMC article. Review.
-
Characterization of carfilzomib-resistant non-small cell lung cancer cell lines.J Cancer Res Clin Oncol. 2018 Jul;144(7):1317-1327. doi: 10.1007/s00432-018-2662-0. Epub 2018 May 15. J Cancer Res Clin Oncol. 2018. PMID: 29766327 Free PMC article.
References
-
- Sartore-Bianchi A, Gasparri F, Galvani A, Nici L, Darnowski JW, Barbone D, Fennell DA, Gaudino G, Porta C, Mutti L. Bortezomib inhibits nuclear factor-kappaB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007;13(19):5942–5951. doi: 10.1158/1078-0432.CCR-07-0536. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

