Combination therapy with docetaxel and S-1 as a first-line treatment in patients with advanced or recurrent gastric cancer: a retrospective analysis
- PMID: 20482840
- PMCID: PMC2885397
- DOI: 10.1186/1477-7819-8-40
Combination therapy with docetaxel and S-1 as a first-line treatment in patients with advanced or recurrent gastric cancer: a retrospective analysis
Abstract
Background: We performed a single-institution retrospective study to evaluate the efficacy and toxicities of combination therapy with docetaxel and S-1 in patients with advanced or recurrent gastric cancer.
Methods: Eighty-six patients with advanced or recurrent gastric cancer were enrolled. Patients received docetaxel, 40 mg/m2, on day 1 and oral S-1, 80 mg/m2/day, on days 1 to 14 every 3 weeks.
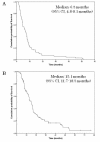
Results: All 84 patients were assessable for response. The overall response rate was 52.4% (44/84) and the disease control rate was 96.4% (81/84). Median time to progression (TTP) and overall survival (OS) were 6.5 (95% CI, 4.8-8.1 months) and 15.1 months (95% CI, 11.7-18.5 months), respectively. The major toxicities were neutropenia, leukopenia, alopecia and anorexia. Grade 3 or 4 hematologic toxicities included neutropenia in 31 patients (36.0%), leukopenia in 27 (31.7%), febrile neutropenia in four (4.7%), and anemia in one (1.2%). Other grade 3 toxicities included anorexia in five patients (5.8%), and stomatitis, diarrhea and nausea in one each (1.2%). There was one treatment-related death (1.2%).
Conclusion: The combination of docetaxel and S-1 had good clinical activity with acceptable toxicity in patients with advanced or recurrent gastric cancer.
Figures
References
-
- Macdonald JS, Schein PS, Woolley PV, Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R, Lagarde C. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med. 1980;93:533–536. - PubMed
-
- Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, Nicolson V, Nash A, Sacks N, Ford H, Carter R, Hill A. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF) Ann Oncol. 1994;5:609–616. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical