Griseofulvin stabilizes microtubule dynamics, activates p53 and inhibits the proliferation of MCF-7 cells synergistically with vinblastine
- PMID: 20482847
- PMCID: PMC2885362
- DOI: 10.1186/1471-2407-10-213
Griseofulvin stabilizes microtubule dynamics, activates p53 and inhibits the proliferation of MCF-7 cells synergistically with vinblastine
Abstract
Background: Griseofulvin, an antifungal drug, has recently been shown to inhibit proliferation of various types of cancer cells and to inhibit tumor growth in athymic mice. Due to its low toxicity, griseofulvin has drawn considerable attention for its potential use in cancer chemotherapy. This work aims to understand how griseofulvin suppresses microtubule dynamics in living cells and sought to elucidate the antimitotic and antiproliferative action of the drug.
Methods: The effects of griseofulvin on the dynamics of individual microtubules in live MCF-7 cells were measured by confocal microscopy. Immunofluorescence microscopy, western blotting and flow cytometry were used to analyze the effects of griseofulvin on spindle microtubule organization, cell cycle progression and apoptosis. Further, interactions of purified tubulin with griseofulvin were studied in vitro by spectrophotometry and spectrofluorimetry. Docking analysis was performed using autodock4 and LigandFit module of Discovery Studio 2.1.
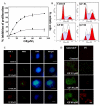
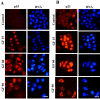
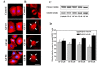
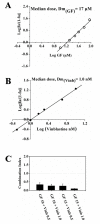
Results: Griseofulvin strongly suppressed the dynamic instability of individual microtubules in live MCF-7 cells by reducing the rate and extent of the growing and shortening phases. At or near half-maximal proliferation inhibitory concentration, griseofulvin dampened the dynamicity of microtubules in MCF-7 cells without significantly disrupting the microtubule network. Griseofulvin-induced mitotic arrest was associated with several mitotic abnormalities like misaligned chromosomes, multipolar spindles, misegregated chromosomes resulting in cells containing fragmented nuclei. These fragmented nuclei were found to contain increased concentration of p53. Using both computational and experimental approaches, we provided evidence suggesting that griseofulvin binds to tubulin in two different sites; one site overlaps with the paclitaxel binding site while the second site is located at the alphabeta intra-dimer interface. In combination studies, griseofulvin and vinblastine were found to exert synergistic effects against MCF-7 cell proliferation.
Conclusions: The study provided evidence suggesting that griseofulvin shares its binding site in tubulin with paclitaxel and kinetically suppresses microtubule dynamics in a similar manner. The results revealed the antimitotic mechanism of action of griseofulvin and provided evidence suggesting that griseofulvin alone and/or in combination with vinblastine may have promising role in breast cancer chemotherapy.
Figures


References
-
- Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, Tseng CJ, Yu CF, Chen RJ, Lin JK. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer. 2001;91:393–401. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1070>3.0.CO;2-#. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

