Thermal stability, pH dependence and inhibition of four murine kynurenine aminotransferases
- PMID: 20482848
- PMCID: PMC2890522
- DOI: 10.1186/1471-2091-11-19
Thermal stability, pH dependence and inhibition of four murine kynurenine aminotransferases
Abstract
Background: Kynurenine aminotransferase (KAT) catalyzes the transamination of kynunrenine to kynurenic acid (KYNA). KYNA is a neuroactive compound and functions as an antagonist of alpha7-nicotinic acetylcholine receptors and is the only known endogenous antagonist of N-methyl-D-aspartate receptors. Four KAT enzymes, KAT I/glutamine transaminase K/cysteine conjugate beta-lyase 1, KAT II/aminoadipate aminotransferase, KAT III/cysteine conjugate beta-lyase 2, and KAT IV/glutamic-oxaloacetic transaminase 2/mitochondrial aspartate aminotransferase, have been reported in mammalian brains. Because of the substrate overlap of the four KAT enzymes, it is difficult to assay the specific activity of each KAT in animal brains.
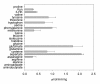
Results: This study concerns the functional expression and comparative characterization of KAT I, II, III, and IV from mice. At the applied test conditions, equimolar tryptophan with kynurenine significantly inhibited only mouse KAT I and IV, equimolar methionine inhibited only mouse KAT III and equimolar aspartate inhibited only mouse KAT IV. The activity of mouse KAT II was not significantly inhibited by any proteinogenic amino acids at equimolar concentrations. pH optima, temperature preferences of four KATs were also tested in this study. Midpoint temperatures of the protein melting, half life values at 65 degrees C, and pKa values of mouse KAT I, II, III, and IV were 69.8, 65.9, 64.8 and 66.5 degrees C; 69.7, 27.4, 3.9 and 6.5 min; pH 7.6, 5.7, 8.7 and 6.9, respectively.
Conclusion: The characteristics reported here could be used to develop specific assay methods for each of the four murine KATs. These specific assays could be used to identify which KAT is affected in mouse models for research and to develop small molecule drugs for prevention and treatment of KAT-involved human diseases.
Figures

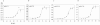
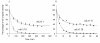


Similar articles
-
Structure, expression, and function of kynurenine aminotransferases in human and rodent brains.Cell Mol Life Sci. 2010 Feb;67(3):353-68. doi: 10.1007/s00018-009-0166-4. Epub 2009 Oct 15. Cell Mol Life Sci. 2010. PMID: 19826765 Free PMC article. Review.
-
Kynurenine aminotransferase III and glutamine transaminase L are identical enzymes that have cysteine S-conjugate β-lyase activity and can transaminate L-selenomethionine.J Biol Chem. 2014 Nov 7;289(45):30950-61. doi: 10.1074/jbc.M114.591461. Epub 2014 Sep 17. J Biol Chem. 2014. PMID: 25231977 Free PMC article.
-
Characterization of rat brain kynurenine aminotransferases I and II.J Neurosci Res. 1997 Nov 1;50(3):457-65. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. J Neurosci Res. 1997. PMID: 9364331
-
Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain.J Neurochem. 2007 Jul;102(1):103-11. doi: 10.1111/j.1471-4159.2007.04556.x. Epub 2007 Apr 17. J Neurochem. 2007. PMID: 17442055
-
The role of glutamine transaminase K (GTK) in sulfur and alpha-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants.Neurochem Int. 2004 Jun;44(8):557-77. doi: 10.1016/j.neuint.2003.12.002. Neurochem Int. 2004. PMID: 15016471 Review.
Cited by
-
Effects of Tianshu Capsule on Spontaneously Hypertensive Rats as Revealed by 1H-NMR-Based Metabolic Profiling.Front Pharmacol. 2019 Sep 11;10:989. doi: 10.3389/fphar.2019.00989. eCollection 2019. Front Pharmacol. 2019. PMID: 31572179 Free PMC article.
-
Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance.Nat Commun. 2019 Jun 24;10(1):2767. doi: 10.1038/s41467-019-10712-0. Nat Commun. 2019. PMID: 31235694 Free PMC article.
-
Tryptophan Metabolism via the Kynurenine Pathway: Implications for Graft Optimization during Machine Perfusion.J Clin Med. 2020 Jun 15;9(6):1864. doi: 10.3390/jcm9061864. J Clin Med. 2020. PMID: 32549246 Free PMC article.
-
Characteristic features of kynurenine aminotransferase allosterically regulated by (alpha)-ketoglutarate in cooperation with kynurenine.PLoS One. 2012;7(7):e40307. doi: 10.1371/journal.pone.0040307. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792273 Free PMC article.
-
Microbiota, Tryptophan and Aryl Hydrocarbon Receptors as the Target Triad in Parkinson's Disease-A Narrative Review.Int J Mol Sci. 2024 Mar 2;25(5):2915. doi: 10.3390/ijms25052915. Int J Mol Sci. 2024. PMID: 38474162 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

