Ultrastructure and molecular phylogenetic position of a novel euglenozoan with extrusive episymbiotic bacteria: Bihospites bacati n. gen. et sp. (Symbiontida)
- PMID: 20482870
- PMCID: PMC2881021
- DOI: 10.1186/1471-2180-10-145
Ultrastructure and molecular phylogenetic position of a novel euglenozoan with extrusive episymbiotic bacteria: Bihospites bacati n. gen. et sp. (Symbiontida)
Abstract
Background: Poorly understood but highly diverse microbial communities exist within anoxic and oxygen-depleted marine sediments. These communities often harbour single-celled eukaryotes that form symbiotic associations with different prokaryotes. During low tides in South-western British Columbia, Canada, vast areas of marine sand become exposed, forming tidal pools. Oxygen-depleted sediments within these pools are distinctively black at only 2-3 cm depth; these layers contain a rich variety of microorganisms, many of which are undescribed. We discovered and characterized a novel (uncultivated) lineage of heterotrophic euglenozoan within these environments using light microscopy, scanning and transmission electron microscopy, serial sectioning and ultrastructural reconstruction, and molecular phylogenetic analyses of small subunit rDNA sequences.
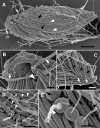
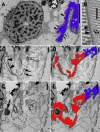
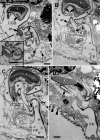
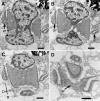
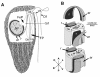
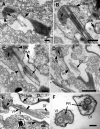
Results: Bihospites bacati n. gen. et sp. is a biflagellated microbial eukaryote that lives within low-oxygen intertidal sands and dies within a few hours of exposure to atmospheric oxygen. The cells are enveloped by two different prokaryotic episymbionts: (1) rod-shaped bacteria and (2) longitudinal strings of spherical bacteria, capable of ejecting an internal, tightly wound thread. Ultrastructural data showed that B. bacati possesses all of the euglenozoan synapomorphies. Moreover, phylogenetic analyses of SSU rDNA sequences demonstrated that B. bacati groups strongly with the Symbiontida: a newly established subclade within the Euglenozoa that includes Calkinsia aureus and other unidentified organisms living in low-oxygen sediments. B. bacati also possessed novel features, such as a compact C-shaped rod apparatus encircling the nucleus, a cytostomal funnel and a distinctive cell surface organization reminiscent of the pellicle strips in phagotrophic euglenids.
Conclusions: We characterized the ultrastructure and molecular phylogenetic position of B. bacati n. gen. et sp. Molecular phylogenetic analyses demonstrated that this species belongs to the Euglenozoa and currently branches as the earliest diverging member of the Symbiontida. This is concordant with ultrastructural features of B. bacati that are intermediate between C. aureus and phagotrophic euglenids, indicating that the most recent ancestor of the Symbiontida descended from phagotrophic euglenids. Additionally, the extrusive episymbionts in B. bacati are strikingly similar to so-called "epixenosomes", prokaryotes previously described in a ciliate species and identified as members of the Verrucomicrobia. These parallel symbioses increase the comparative context for understanding the origin(s) of extrusive organelles in eukaryotes and underscores how little we know about the symbiotic communities of marine benthic environments.
Figures






References
-
- Triemer RE, Farmer MA. In: The Biology of Free-living Heterotrophic Flagellates. Patterson DJ, Larsen J, editor. Clarendon Press, Oxford; 1991. The ultrastructural organization of the heterotrophic euglenids and its evolutionary implications; pp. 205–217.
-
- Leander BS, Triemer RE, Farmer MA. Character evolution in heterotrophic euglenids. Eur J Protistol. 2001;37:337–356. doi: 10.1078/0932-4739-00842. - DOI
-
- Simpson AGB, Lukes J, Roger AJ. The evolutionary history of kinetoplastids and their kinetoplasts. Mol Biol Evol. 2002;19:2071–2083. - PubMed
-
- Kivic PA, Walne PL. An evaluation of the possible phylogenetic relationship between euglenophyta and kinetoplastida. Orig Life. 1984;13:269–288. doi: 10.1007/BF00927177. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

