Mechanisms controlling cell size and shape during isotropic cell spreading
- PMID: 20483321
- PMCID: PMC2872297
- DOI: 10.1016/j.bpj.2010.01.059
Mechanisms controlling cell size and shape during isotropic cell spreading
Erratum in
- Biophys J. 2010 Jul 21;99(2):695
Abstract
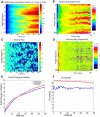
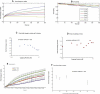
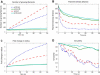
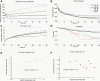
Cell motility is important for many developmental and physiological processes. Motility arises from interactions between physical forces at the cell surface membrane and the biochemical reactions that control the actin cytoskeleton. To computationally analyze how these factors interact, we built a three-dimensional stochastic model of the experimentally observed isotropic spreading phase of mammalian fibroblasts. The multiscale model is composed at the microscopic levels of three actin filament remodeling reactions that occur stochastically in space and time, and these reactions are regulated by the membrane forces due to membrane surface resistance (load) and bending energy. The macroscopic output of the model (isotropic spreading of the whole cell) occurs due to the movement of the leading edge, resulting solely from membrane force-constrained biochemical reactions. Numerical simulations indicate that our model qualitatively captures the experimentally observed isotropic cell-spreading behavior. The model predicts that increasing the capping protein concentration will lead to a proportional decrease in the spread radius of the cell. This prediction was experimentally confirmed with the use of Cytochalasin D, which caps growing actin filaments. Similarly, the predicted effect of actin monomer concentration was experimentally verified by using Latrunculin A. Parameter variation analyses indicate that membrane physical forces control cell shape during spreading, whereas the biochemical reactions underlying actin cytoskeleton dynamics control cell size (i.e., the rate of spreading). Thus, during cell spreading, a balance between the biochemical and biophysical properties determines the cell size and shape. These mechanistic insights can provide a format for understanding how force and chemical signals together modulate cellular regulatory networks to control cell motility.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Signaling network triggers and membrane physical properties control the actin cytoskeleton-driven isotropic phase of cell spreading.Biophys J. 2011 Feb 16;100(4):845-57. doi: 10.1016/j.bpj.2010.12.3732. Biophys J. 2011. PMID: 21320428 Free PMC article.
-
Force transmission in migrating cells.J Cell Biol. 2010 Jan 25;188(2):287-97. doi: 10.1083/jcb.200906139. J Cell Biol. 2010. PMID: 20100912 Free PMC article.
-
Quantification of cell edge velocities and traction forces reveals distinct motility modules during cell spreading.PLoS One. 2008;3(11):e3735. doi: 10.1371/journal.pone.0003735. Epub 2008 Nov 17. PLoS One. 2008. PMID: 19011687 Free PMC article.
-
Control of polarized assembly of actin filaments in cell motility.Cell Mol Life Sci. 2015 Aug;72(16):3051-67. doi: 10.1007/s00018-015-1914-2. Epub 2015 May 7. Cell Mol Life Sci. 2015. PMID: 25948416 Free PMC article. Review.
-
Actin, a central player in cell shape and movement.Science. 2009 Nov 27;326(5957):1208-12. doi: 10.1126/science.1175862. Science. 2009. PMID: 19965462 Free PMC article. Review.
Cited by
-
Mathematical modeling of the impact of actin and keratin filaments on keratinocyte cell spreading.Biophys J. 2012 Nov 7;103(9):1828-38. doi: 10.1016/j.bpj.2012.09.016. Biophys J. 2012. PMID: 23199911 Free PMC article.
-
Universal Kinetics of the Onset of Cell Spreading on Substrates of Different Stiffness.Biophys J. 2019 Feb 5;116(3):551-559. doi: 10.1016/j.bpj.2018.12.020. Epub 2019 Jan 5. Biophys J. 2019. PMID: 30665696 Free PMC article.
-
Systems biology of cellular membranes: a convergence with biophysics.Wiley Interdiscip Rev Syst Biol Med. 2017 Sep;9(5):10.1002/wsbm.1386. doi: 10.1002/wsbm.1386. Epub 2017 May 5. Wiley Interdiscip Rev Syst Biol Med. 2017. PMID: 28475297 Free PMC article. Review.
-
A mathematical model of the coupled mechanisms of cell adhesion, contraction and spreading.J Math Biol. 2014 Mar;68(4):989-1022. doi: 10.1007/s00285-013-0656-8. Epub 2013 Mar 6. J Math Biol. 2014. PMID: 23463540 Free PMC article.
-
Multiscale modeling of cell shape from the actin cytoskeleton.Prog Mol Biol Transl Sci. 2014;123:143-67. doi: 10.1016/B978-0-12-397897-4.00002-4. Prog Mol Biol Transl Sci. 2014. PMID: 24560144 Free PMC article. Review.
References
-
- Pollard T.D., Berro J. Mathematical models and simulations of cellular processes based on actin filaments. J. Biol. Chem. 2009;284:5433–5437. - PubMed
-
- Döbereiner H.G., Dubin-Thaler B., Sheetz M.P. Dynamic phase transitions in cell spreading. Phys. Rev. Lett. 2004;93:108105. - PubMed
-
- Loisel T.P., Boujemaa R., Carlier M.F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

