Independent and cooperative motions of the Kv1.2 channel: voltage sensing and gating
- PMID: 20483326
- PMCID: PMC2872368
- DOI: 10.1016/j.bpj.2010.01.049
Independent and cooperative motions of the Kv1.2 channel: voltage sensing and gating
Abstract
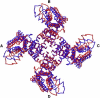
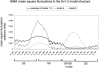
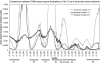
Voltage-gated potassium (Kv) channels, such as Kv1.2, are involved in the generation and propagation of action potentials. The Kv channel is a homotetramer, and each monomer is composed of a voltage-sensing domain (VSD) and a pore domain (PD). We analyzed the fluctuations of a model structure of Kv1.2 using elastic network models. The analysis suggested a network of coupled fluctuations of eight rigid structural units and seven hinges that may control the transition between the active and inactive states of the channel. For the most part, the network is composed of amino acids that are known to affect channel activity. The results suggested allosteric interactions and cooperativity between the subunits in the coupling between the motion of the VSD and the selectivity filter of the PD, in accordance with recent empirical data. There are no direct contacts between the VSDs of the four subunits, and the contacts between these and the PDs are loose, suggesting that the VSDs are capable of functioning independently. Indeed, they manifest many inherent fluctuations that are decoupled from the rest of the structure. In general, the analysis suggests that the two domains contribute to the channel function both individually and cooperatively.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

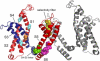

Similar articles
-
Structure prediction for the down state of a potassium channel voltage sensor.Nature. 2007 Feb 1;445(7127):550-3. doi: 10.1038/nature05494. Epub 2006 Dec 24. Nature. 2007. PMID: 17187053
-
Molecular mechanism for depolarization-induced modulation of Kv channel closure.J Gen Physiol. 2012 Nov;140(5):481-93. doi: 10.1085/jgp.201210817. Epub 2012 Oct 15. J Gen Physiol. 2012. PMID: 23071266 Free PMC article.
-
Moving gating charges through the gating pore in a Kv channel voltage sensor.Proc Natl Acad Sci U S A. 2014 May 13;111(19):E1950-9. doi: 10.1073/pnas.1406161111. Epub 2014 Apr 29. Proc Natl Acad Sci U S A. 2014. PMID: 24782544 Free PMC article.
-
Dissecting the coupling between the voltage sensor and pore domains.Neuron. 2006 Nov 22;52(4):568-9. doi: 10.1016/j.neuron.2006.11.002. Neuron. 2006. PMID: 17114039 Review.
-
Omega currents in voltage-gated ion channels: what can we learn from uncovering the voltage-sensing mechanism using MD simulations?Acc Chem Res. 2013 Dec 17;46(12):2755-62. doi: 10.1021/ar300290u. Epub 2013 May 22. Acc Chem Res. 2013. PMID: 23697886 Review.
Cited by
-
Structure and flexibility of the C-ring in the electromotor of rotary F(0)F(1)-ATPase of pea chloroplasts.PLoS One. 2012;7(9):e43045. doi: 10.1371/journal.pone.0043045. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049735 Free PMC article.
-
Structure-Encoded Global Motions and Their Role in Mediating Protein-Substrate Interactions.Biophys J. 2015 Sep 15;109(6):1101-9. doi: 10.1016/j.bpj.2015.06.004. Epub 2015 Jul 2. Biophys J. 2015. PMID: 26143655 Free PMC article. Review.
-
Structure, dynamics and implied gating mechanism of a human cyclic nucleotide-gated channel.PLoS Comput Biol. 2014 Dec 4;10(12):e1003976. doi: 10.1371/journal.pcbi.1003976. eCollection 2014 Dec. PLoS Comput Biol. 2014. PMID: 25474149 Free PMC article.
-
General rules for the arrangements and gating motions of pore-lining helices in homomeric ion channels.Nat Commun. 2014 Aug 8;5:4641. doi: 10.1038/ncomms5641. Nat Commun. 2014. PMID: 25105557 Free PMC article.
-
Dynamic allostery: linkers are not merely flexible.Structure. 2011 Jul 13;19(7):907-17. doi: 10.1016/j.str.2011.06.002. Structure. 2011. PMID: 21742258 Free PMC article. Review.
References
-
- Sanguinetti M.C., Spector P.S. Potassium channelopathies. Neuropharmacology. 1997;36:755–762. - PubMed
-
- Jiang Y., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. - PubMed
-
- Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
-
- Pathak M.M., Yarov-Yarovoy V., Isacoff E.Y. Closing in on the resting state of the Shaker K(+) channel. Neuron. 2007;56:124–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

