Understanding detergent effects on lipid membranes: a model study of lysolipids
- PMID: 20483328
- PMCID: PMC2872274
- DOI: 10.1016/j.bpj.2010.01.037
Understanding detergent effects on lipid membranes: a model study of lysolipids
Abstract
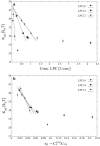
Lysolipids and fatty acids are the natural products formed by the hydrolysis of phospholipids. Lysolipids and fatty acids form micelles in solution and acts as detergents in the presence of lipid membranes. In this study, we investigate the detergent strength of a homologous series of lyso-phosphatidylcholine lipids (LPCs) on 1-palmitoyl-2-oleyl-sn-glycerol-3-phosphatidylcholine (POPC) lipid membranes by use of isothermal titration calorimetry and vesicle fluctuation analysis. The membrane partition coefficient (K) and critical micelle concentration (cmc) are determined by isothermal titration calorimetry and found to obey an inverse proportionality relation (cmc.K approximately 0.05-0.3). The partition coefficient and critical micelle concentration are used for the analysis of the effect of LPCs on the membrane bending rigidity. The dependency of the bending rigidity on LPC membrane coverage has been analyzed in terms of a phenomenological model based on continuum elastic theory, which yields information about the curvature-inducing properties of the LPC molecule. The results reveal: 1), an increase in the partition coefficient with increasing LPC acyl-chain length; and 2), that the degree of acyl-chain mismatch between LPC and POPC determines the magnitude of the membrane mechanical perturbation per LPC molecule in the membrane. Finally, the three-stage model describing detergent membrane interaction has been extended by a parameter D(MCI), which governs the membrane curvature stability in the detergent concentration range below the cmc-value of the LPC molecule.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Naturforsch Z. 1973;28:693–703. - PubMed
-
- de Gennes P.G., Taupin C. Microemulsions and the flexibility of oil/water interfaces. J. Phys. Chem. 1982;86:2294–2304.
-
- Koynova R., Tenchov B. Interaction of surfactants and fatty acids with lipids. Curr. Opin. Colloid Interface Sci. 2001;6:277–286.
-
- Alonso, A., and F. Goni. (Guest editors.). 2000. Detergents in biomembranes studies. Biochim. Biophys. Acta. 1508:1–19. (Special issue). - PubMed
-
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim. Biophys. Acta. 1975;415:29–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

