Influence of the lipid anchor motif of N-ras on the interaction with lipid membranes: a surface plasmon resonance study
- PMID: 20483331
- PMCID: PMC2872271
- DOI: 10.1016/j.bpj.2010.02.005
Influence of the lipid anchor motif of N-ras on the interaction with lipid membranes: a surface plasmon resonance study
Abstract
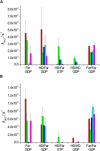
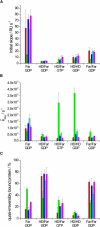
Ras GTPases play a crucial role in signal transduction cascades involved in cell differentiation and proliferation, and membrane binding is essential for their proper function. To determine the influence of the nature of the lipid anchor motif and the difference between the active (GTP) and inactive (GDP) forms of N-Ras on partitioning and localization in the lipid membrane, five different N-Ras constructs with different lipid anchors and nucleotide loading (Far/Far (GDP), HD/Far (GDP), HD/HD (GDP), Far (GDP), and HD/Far (GppNHp)) were synthesized. Using the surface plasmon resonance technique, we were able to follow the insertion and dissociation process of the lipidated proteins into and out of model membranes consisting of pure liquid-ordered (l(o)) or liquid-disordered (l(d)) phase and a heterogeneous two-phase mixture, i.e., a raft mixture with l(o) + l(d) phase coexistence. In addition, we examined the influence of negatively charged headgroups and stored curvature elastic stress on the binding properties of the lipidated N-Ras proteins. In most cases, significant differences were found for the various anchor motifs. In general, N-Ras proteins insert preferentially into a fluidlike, rather than a rigid, ordered lipid bilayer environment. Electrostatic interactions with lipid headgroups or stored curvature elastic stress of the membrane seem to have no drastic effect on the binding and dissociation processes of the lipidated proteins. The monofarnesylated N-Ras exhibits generally the highest association rate and fastest dissociation process in fluidlike membranes. Double lipidation, especially including farnesylation, of the protein leads to drastically reduced initial binding rates but strong final association. The change in the nucleotide loading of the natural N-Ras HD/Far induces a slightly different binding and dissociation kinetics, as well as stability of association, and seems to influence the tendency to segregate laterally in the membrane plane. The GDP-bound inactive form of N-Ras with an HD/Far anchor shows stronger membrane association, which might be due to a more pronounced tendency to self-assemble in the membrane matrix than is seen with the active GTP-bound form.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Membrane-mediated induction and sorting of K-Ras microdomain signaling platforms.J Am Chem Soc. 2011 Feb 2;133(4):880-7. doi: 10.1021/ja107532q. Epub 2010 Dec 9. J Am Chem Soc. 2011. PMID: 21141956
-
Visualizing association of lipidated signaling proteins in heterogeneous membranes--partitioning into subdomains, lipid sorting, interfacial adsorption, and protein association.Biochim Biophys Acta. 2010 Jul;1798(7):1409-17. doi: 10.1016/j.bbamem.2009.12.006. Epub 2009 Dec 16. Biochim Biophys Acta. 2010. PMID: 20025847
-
Study of Förster Resonance Energy Transfer to Lipid Domain Markers Ascertains Partitioning of Semisynthetic Lipidated N-Ras in Lipid Raft Nanodomains.Biochemistry. 2018 Feb 6;57(5):872-881. doi: 10.1021/acs.biochem.7b01181. Epub 2018 Jan 10. Biochemistry. 2018. PMID: 29280621
-
GTP hydrolysis mechanism of Ras-like GTPases.J Mol Biol. 2004 Jul 23;340(5):921-32. doi: 10.1016/j.jmb.2004.06.007. J Mol Biol. 2004. PMID: 15236956 Review.
-
Influence of isoform-specific Ras lipidation motifs on protein partitioning and dynamics in model membrane systems of various complexity.Biol Chem. 2017 May 1;398(5-6):547-563. doi: 10.1515/hsz-2016-0289. Biol Chem. 2017. PMID: 27977396 Review.
Cited by
-
Binding mechanism of the matrix domain of HIV-1 gag on lipid membranes.Elife. 2020 Aug 18;9:e58621. doi: 10.7554/eLife.58621. Elife. 2020. PMID: 32808928 Free PMC article.
-
Regulation of K-Ras4B Membrane Binding by Calmodulin.Biophys J. 2016 Jul 12;111(1):113-22. doi: 10.1016/j.bpj.2016.05.042. Biophys J. 2016. PMID: 27410739 Free PMC article.
-
The intracellular dynamic of protein palmitoylation.J Cell Biol. 2010 Dec 27;191(7):1229-38. doi: 10.1083/jcb.201008160. J Cell Biol. 2010. PMID: 21187327 Free PMC article. Review.
-
Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus.Antimicrob Agents Chemother. 2014 Nov;58(11):6819-27. doi: 10.1128/AAC.00061-14. Epub 2014 Sep 2. Antimicrob Agents Chemother. 2014. PMID: 25182650 Free PMC article.
-
Lipid packing defects and membrane charge control RAB GTPase recruitment.Traffic. 2018 Jul;19(7):536-545. doi: 10.1111/tra.12568. Epub 2018 Apr 6. Traffic. 2018. PMID: 29573133 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

