Amyloid-beta fibrillogenesis seeded by interface-induced peptide misfolding and self-assembly
- PMID: 20483339
- PMCID: PMC2872264
- DOI: 10.1016/j.bpj.2010.01.056
Amyloid-beta fibrillogenesis seeded by interface-induced peptide misfolding and self-assembly
Abstract
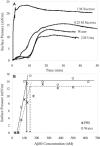
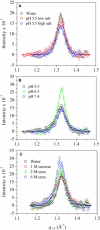
The amphipathicity of the natively unstructured amyloid-beta (Abeta40) peptide may play an important role in its aggregation into beta-sheet rich fibrils, which is linked to the pathogenesis of Alzheimer's disease. Using the air/subphase interface as a model interface, we characterized Abeta's surface activity and its conformation, assembly, and morphology at the interface. Abeta readily adsorbed to the air/subphase interface to form a 20 A thick film and showed a critical micelle concentration of approximately 120 nM. Abeta adsorbed at the air/subphase exhibited in-plane ordering that gave rise to Bragg peaks in grazing-incidence x-ray diffraction measurements. Analysis of the peaks showed that the air/subphase interface induced Abeta to fold into a beta-sheet conformation and to self-assemble into approximately 100 A-sized ordered clusters. The formation of these clusters at the air/subphase interface was not affected by pH, salts, or the presence of sucrose or urea, which are known to stabilize or denature native proteins, suggesting that interface-driven Abeta misfolding and assembly are strongly favored. Furthermore, Abeta at the interface seeded the growth of fibrils in the bulk with a distinct morphology compared to those formed by homogeneous nucleation. Our results indicate that interface-induced Abeta misfolding may serve as a heterogeneous, nucleation-controlled aggregation mechanism for Abeta fibrillogenesis in vivo.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Chi E.Y., Frey S.L., Lee K.Y.C. Ganglioside G(M1)-mediated amyloid-β fibrillogenesis and membrane disruption. Biochemistry. 2007;46:1913–1924. - PubMed
-
- Sabaté R., Estelrich J. Evidence of the existence of micelles in the fibrillogenesis of β-amyloid peptide. J. Phys. Chem. B. 2005;109:11027–11032. - PubMed
-
- Soreghan B., Kosmoski J., Glabe C. Surfactant properties of Alzheimer's Aβ peptides and the mechanism of amyloid aggregation. J. Biol. Chem. 1994;269:28551–28554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

