Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles
- PMID: 20483366
- PMCID: PMC2914110
- DOI: 10.1016/j.jconrel.2010.05.003
Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles
Abstract
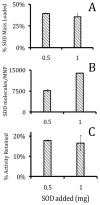
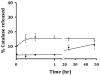
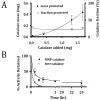
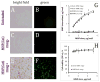
Antioxidant enzymes have shown promise as a therapy for pathological conditions involving increased production of reactive oxygen species (ROS). However the efficiency of their use for combating oxidative stress is dependent on the ability to achieve therapeutically adequate levels of active enzymes at the site of ROS-mediated injury. Thus, the implementation of antioxidant enzyme therapy requires a strategy enabling both guided delivery to the target site and effective protection of the protein in its active form. To address these requirements we developed magnetically responsive nanoparticles (MNP) formed by precipitation of calcium oleate in the presence of magnetite-based ferrofluid (controlled aggregation/precipitation) as a carrier for magnetically guided delivery of therapeutic proteins. We hypothesized that antioxidant enzymes, catalase and superoxide dismutase (SOD), can be protected from proteolytic inactivation by encapsulation in MNP. We also hypothesized that catalase-loaded MNP applied with a high-gradient magnetic field can rescue endothelial cells from hydrogen peroxide toxicity in culture. To test these hypotheses, a family of enzyme-loaded MNP formulations were prepared and characterized with respect to their magnetic properties, enzyme entrapment yields and protection capacity. SOD- and catalase-loaded MNP were formed with average sizes ranging from 300 to 400 nm, and a protein loading efficiency of 20-33%. MNP were strongly magnetically responsive (magnetic moment at saturation of 14.3 emu/g) in the absence of magnetic remanence, and exhibited a protracted release of their cargo protein in plasma. Catalase stably associated with MNP was protected from proteolysis and retained 20% of its initial enzymatic activity after 24h of exposure to pronase. Under magnetic guidance catalase-loaded MNP were rapidly taken up by cultured endothelial cells providing increased resistance to oxidative stress (62+/-12% cells rescued from hydrogen peroxide induced cell death vs. 10+/-4% under non-magnetic conditions). We conclude that non-polymeric MNP formed using the controlled aggregation/precipitation strategy are a promising carrier for targeted antioxidant enzyme therapy, and in combination with magnetic guidance can be applied to protect endothelial cells from oxidative stress mediated damage. This protective effect of magnetically targeted MNP impregnated with antioxidant enzymes can be highly relevant for the treatment of cardiovascular disease and should be further investigated in animal models.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064–2110. - PubMed
-
- Namdeo M, Saxena S, Tankhiwale R, Bajpai M, Mohan YM, Bajpai SK. Magnetic nanoparticles for drug delivery applications. J Nanosci Nanotechnol. 2008;8(7):3247–3271. - PubMed
-
- Ma HL, Qi XR, Ding WX, Maitani Y, Nagai T. Magnetic targeting after femoral artery administration and biocompatibility assessment of superparamagnetic iron oxide nanoparticles. J Biomed Mater Res A. 2008;84(3):598–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

