The roles of IL-12 and IL-23 in CD8+ T cell-mediated immunity against Listeria monocytogenes: Insights from a DC vaccination model
- PMID: 20483409
- PMCID: PMC2902594
- DOI: 10.1016/j.cellimm.2010.04.007
The roles of IL-12 and IL-23 in CD8+ T cell-mediated immunity against Listeria monocytogenes: Insights from a DC vaccination model
Abstract
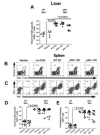
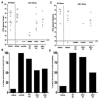
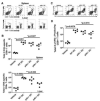
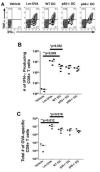
Listeria monocytogenes infection induces a strong inflammatory response characterized by the production of IL-12 and IFN-gamma and protective immunity against this pathogen is dependent on CD8+ T cells (CTL). Recent studies have suggested that these inflammatory cytokines affect the rate of memory CD8+ T cell generation as well as the number of short-lived effector cells generated. The role of the closely related cytokine, IL-23, in this response has not been examined. We hypothesized that IL-12 and IL-23 produced by dendritic cells collectively enhance the generation and function of memory cells. To test this hypothesis, we employed a DC vaccination approach. Mice lacking IL-12 and IL-23 were vaccinated with wild-type (WT), IL-12(-/-), or IL-12/23(-/-) DC and protection to Lm was monitored. Mice vaccinated with WT and IL-12(-/-) DC were resistant to lethal challenge with Lm. Surprisingly, mice vaccinated with IL-12/23(-/-) DC exhibited significantly reduced protection when challenged. Protection correlated with the relative size of the memory pools generated. In summary, these data indicate that IL-23 can partially compensate for the lack of IL-12 in the generation protective immunity against Lm.
2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes.J Immunol. 2007 Aug 15;179(4):2457-66. doi: 10.4049/jimmunol.179.4.2457. J Immunol. 2007. PMID: 17675507
-
Mucosal CD8 T Cell Responses Are Shaped by Batf3-DC After Foodborne Listeria monocytogenes Infection.Front Immunol. 2020 Sep 11;11:575967. doi: 10.3389/fimmu.2020.575967. eCollection 2020. Front Immunol. 2020. PMID: 33042159 Free PMC article.
-
MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria.Immunology. 2009 Nov;128(3):429-38. doi: 10.1111/j.1365-2567.2009.03128.x. Immunology. 2009. PMID: 20067542 Free PMC article.
-
Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes.J Immunol. 2005 Aug 1;175(3):1751-7. doi: 10.4049/jimmunol.175.3.1751. J Immunol. 2005. PMID: 16034116 Free PMC article.
-
Probing CD8 T cell responses with Listeria monocytogenes infection.Adv Immunol. 2012;113:51-80. doi: 10.1016/B978-0-12-394590-7.00005-1. Adv Immunol. 2012. PMID: 22244579 Review.
Cited by
-
MERTK inhibition alters the PD-1 axis and promotes anti-leukemia immunity.JCI Insight. 2018 Nov 2;3(21):e97941. doi: 10.1172/jci.insight.97941. JCI Insight. 2018. PMID: 30385715 Free PMC article.
-
The intact structural form of LLO in endosomes cannot protect against listeriosis.Int J Biochem Mol Biol. 2011;2(3):207-18. Epub 2011 Jul 15. Int J Biochem Mol Biol. 2011. PMID: 22003433 Free PMC article.
-
The IL-23/Th17 axis is involved in the adaptive immune response to Bacillus anthracis in humans.Eur J Immunol. 2014 Mar;44(3):752-62. doi: 10.1002/eji.201343784. Epub 2014 Jan 2. Eur J Immunol. 2014. PMID: 24643777 Free PMC article.
-
Illuminating the petite picture of T cell memory responses to Listeria monocytogenes.Biomed Res Int. 2013;2013:121684. doi: 10.1155/2013/121684. Epub 2013 Sep 22. Biomed Res Int. 2013. PMID: 24171157 Free PMC article. Review.
-
Maturation of morphology, phenotype and functions of murine bone marrow-derived dendritic cells (DCs) induced by polysaccharide Kureha (PSK).Hum Vaccin Immunother. 2012 Dec 1;8(12):1808-16. doi: 10.4161/hv.21993. Epub 2012 Oct 2. Hum Vaccin Immunother. 2012. PMID: 23032163 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

