Device Thrombogenicity Emulator (DTE)--design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs
- PMID: 20483411
- PMCID: PMC2922449
- DOI: 10.1016/j.jbiomech.2010.04.020
Device Thrombogenicity Emulator (DTE)--design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs
Abstract
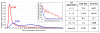
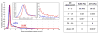
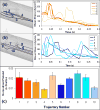
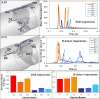
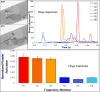
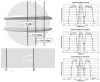
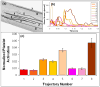
Patients who receive prosthetic heart valve (PHV) implants require mandatory anticoagulation medication after implantation due to the thrombogenic potential of the valve. Optimization of PHV designs may facilitate reduction of flow-induced thrombogenicity and reduce or eliminate the need for post-implant anticoagulants. We present a methodology entitled Device Thrombogenicty Emulator (DTE) for optimizing the thrombo-resistance performance of PHV by combining numerical and experimental approaches. Two bileaflet mechanical heart valves (MHV) designs, St. Jude Medical (SJM) and ATS, were investigated by studying the effect of distinct flow phases on platelet activation. Transient turbulent and direct numerical simulations (DNS) were conducted, and stress loading histories experienced by the platelets were calculated along flow trajectories. The numerical simulations indicated distinct design dependent differences between the two valves. The stress loading waveforms extracted from the numerical simulations were programmed into a hemodynamic shearing device (HSD), emulating the flow conditions past the valves in distinct 'hot-spot' flow regions that are implicated in MHV thrombogenicity. The resultant platelet activity was measured with a modified prothrombinase assay, and was found to be significantly higher in the SJM valve, mostly during the regurgitation phase. The experimental results were in excellent agreement with the calculated platelet activation potential. This establishes the utility of the DTE methodology for serving as a test bed for evaluating design modifications for achieving better thrombogenic performance for such devices.
2010 Elsevier Ltd. All rights reserved.
Figures







References
-
- Alemu Y, Bluestein D. Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artificial Organs. 2007;31(9):677–688. - PubMed
-
- Apel J, Neudel F, Reul H. Computational fluid dynamics and experimental validation of a microaxial blood pump. ASAIO Journal. 2001;47(5):552–558. - PubMed
-
- Arora D, Behr M, Pasquali M. A Tensor-based Measure for Estimating Blood Damage. Artificial Organs. 2004;28(11):1002–1015. - PubMed
-
- Bernet FH, Baykut D, Grize L, Zerkowski HR. Single-center outcome analysis of 1,161 patients with St. Jude medical and ATS open pivot mechanical heart valves. Journal of Heart Valve Disease. 2007;16(2):151–158. - PubMed
-
- Bludszuweit C. Model for a general mechanical blood damage prediction. Artificial Organs. 1995;19(7):583–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

