Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[(11)C]DASB and structural brain imaging study
- PMID: 20483717
- PMCID: PMC2912692
- DOI: 10.1093/brain/awq103
Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[(11)C]DASB and structural brain imaging study
Abstract
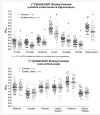
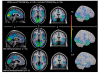
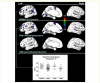
Animal data indicate that the recreational drug ecstasy (3,4-methylenedioxymethamphetamine) can damage brain serotonin neurons. However, human neuroimaging measurements of serotonin transporter binding, a serotonin neuron marker, remain contradictory, especially regarding brain areas affected; and the possibility that structural brain differences might account for serotonin transporter binding changes has not been explored. We measured brain serotonin transporter binding using [(11)C] N,N-dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine in 50 control subjects and in 49 chronic (mean 4 years) ecstasy users (typically one to two tablets bi-monthly) withdrawn from the drug (mean 45 days). A magnetic resonance image for positron emission tomography image co-registration and structural analyses was acquired. Hair toxicology confirmed group allocation but also indicated use of other psychoactive drugs in most users. Serotonin transporter binding in ecstasy users was significantly decreased throughout all cerebral cortices (range -19 to -46%) and hippocampus (-21%) and related to the extent of drug use (years, maximum dose), but was normal in basal ganglia and midbrain. Substantial overlap was observed between control and user values except for insular cortex, in which 51% of ecstasy user values fell below the lower limit of the control range. Voxel-based analyses confirmed a caudorostral gradient of cortical serotonin transporter binding loss with occipital cortex most severely affected. Magnetic resonance image measurement revealed no overall regional volume differences between groups; however, a slight left-hemispheric biased cortical thinning was detected in methamphetamine-using ecstasy users. The serotonin transporter binding loss was not related to structural changes or partial volume effect, use of other stimulant drugs, blood testosterone or oestradiol levels, major serotonin transporter gene promoter polymorphisms, gender, psychiatric status, or self-reported hyperthermia or tolerance. The ecstasy group, although 'grossly behaviourally normal', reported subnormal mood and demonstrated generally modest deficits on some tests of attention, executive function and memory, with the latter associated with serotonin transporter decrease. Our findings suggest that the 'typical'/low dose (one to two tablets/session) chronic ecstasy-polydrug user might display a highly selective mild to marked loss of serotonin transporter in cerebral cortex/hippocampus in the range of that observed in Parkinson's disease, which is not gender-specific or completely accounted for by structural brain changes, recent use of other drugs (as assessed by hair analyses) or other potential confounds that we could address. The striking sparing of serotonin transporter-rich striatum (although possibly affected in 'heavier' users) suggests that serotonergic neurons innervating cerebral cortex are more susceptible, for unknown reasons, to ecstasy than those innervating subcortical regions and that behavioural problems in some ecstasy users during abstinence might be related to serotonin transporter changes limited to cortical regions.
Figures
Similar articles
-
In vivo imaging of cerebral serotonin transporter and serotonin(2A) receptor binding in 3,4-methylenedioxymethamphetamine (MDMA or "ecstasy") and hallucinogen users.Arch Gen Psychiatry. 2011 Jun;68(6):562-76. doi: 10.1001/archgenpsychiatry.2011.56. Arch Gen Psychiatry. 2011. PMID: 21646575
-
Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users.Psychopharmacology (Berl). 2003 Apr;167(1):85-96. doi: 10.1007/s00213-002-1383-9. Epub 2003 Mar 11. Psychopharmacology (Berl). 2003. PMID: 12632248
-
Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective.J Psychopharmacol. 2006 Mar;20(2):211-25. doi: 10.1177/0269881106059486. J Psychopharmacol. 2006. PMID: 16510479 Review.
-
Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/-)3,4-methylenedioxymethamphetamine ("ecstasy") users: relationship to cognitive performance.Psychopharmacology (Berl). 2008 Oct;200(3):439-50. doi: 10.1007/s00213-008-1218-4. Epub 2008 Jul 27. Psychopharmacology (Berl). 2008. PMID: 18661256 Free PMC article.
-
The effects of ecstasy on neurotransmitter systems: a review on the findings of molecular imaging studies.Psychopharmacology (Berl). 2016 Oct;233(19-20):3473-501. doi: 10.1007/s00213-016-4396-5. Epub 2016 Aug 28. Psychopharmacology (Berl). 2016. PMID: 27568200 Free PMC article. Review.
Cited by
-
MDMA 'ecstasy' increases cerebral cortical perfusion determined by bolus-tracking arterial spin labelling (btASL) MRI.Br J Pharmacol. 2013 Jul;169(5):974-87. doi: 10.1111/bph.12178. Br J Pharmacol. 2013. PMID: 23517012 Free PMC article.
-
Sustained recreational use of ecstasy is associated with altered pre and postsynaptic markers of serotonin transmission in neocortical areas: a PET study with [¹¹C]DASB and [¹¹C]MDL 100907.Neuropsychopharmacology. 2012 May;37(6):1465-73. doi: 10.1038/npp.2011.332. Epub 2012 Feb 22. Neuropsychopharmacology. 2012. PMID: 22353758 Free PMC article.
-
Evidence for chronically altered serotonin function in the cerebral cortex of female 3,4-methylenedioxymethamphetamine polydrug users.Arch Gen Psychiatry. 2012 Apr;69(4):399-409. doi: 10.1001/archgenpsychiatry.2011.156. Epub 2011 Dec 5. Arch Gen Psychiatry. 2012. PMID: 22147810 Free PMC article.
-
5-HTTLPR Genotype Moderates the Effects of Past Ecstasy Use on Verbal Memory Performance in Adolescent and Emerging Adults: A Pilot Study.PLoS One. 2015 Jul 31;10(7):e0134708. doi: 10.1371/journal.pone.0134708. eCollection 2015. PLoS One. 2015. PMID: 26231032 Free PMC article.
-
3,4-methylenedioxymethamphetamine (MDMA): current perspectives.Subst Abuse Rehabil. 2013 Nov 21;4:83-99. doi: 10.2147/SAR.S37258. eCollection 2013. Subst Abuse Rehabil. 2013. PMID: 24648791 Free PMC article. Review.
References
-
- Ad-Dab'bagh Y, Einarson D, Lyttelton O, Muehlboeck JS, Mok K, Ivanov O, et al. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: NeuroImage, editor. Organization for Human Brain Mapping. Italy: Florence; 2006.
-
- Ad-Dab'bagh Y, Singh V, Robbins S, Lerch J, Lyttelton O, Fombonne E, et al. Native space cortical thickness measurement and the absence of correlation to cerebral volume. In: Zilles K, editor. Organization for human brain mapping. Toronto, Canada: Neuroimage; 2005.
-
- Bailey DL, Young H, Bloomfield PM, Meikle SR, Glass D, Myers MJ, et al. ECAT ART—a continuously rotating PET camera: performance characteristics, initial clinical studies, and installation considerations in a nuclear medicine department. Eur J Nucl Med. 1997;24:6–15. - PubMed
-
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, et al. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson's disease. Brain. 2009;132:1366–75. - PubMed
-
- Bouso JC, Doblin R, Farre M, Alcazar MA, Gomez-Jarabo G. MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. J Psychoactive Drugs. 2008;40:225–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

