Dendritic cell-directed CTLA-4 engagement during pancreatic beta cell antigen presentation delays type 1 diabetes
- PMID: 20483724
- PMCID: PMC2882504
- DOI: 10.4049/jimmunol.0903130
Dendritic cell-directed CTLA-4 engagement during pancreatic beta cell antigen presentation delays type 1 diabetes
Abstract
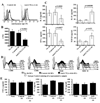
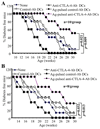
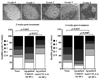
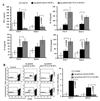
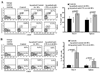
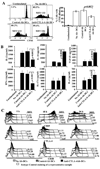
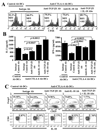
The levels of expression of alternatively spliced variants of CTLA-4 and insufficient CTLA-4 signaling have been implicated in type 1 diabetes. Hence, we hypothesized that increasing CTLA-4-specific ligand strength on autoantigen-presenting dendritic cells (DCs) can enhance ligation of CTLA-4 on T cells and lead to modulation of autoreactive T cell response. In this study, we show that DC-directed enhanced CTLA-4 engagement upon pancreatic beta cell Ag presentation results in the suppression of autoreactive T cell response in NOD mice. The T cells from prediabetic NOD mice treated with an agonistic anti-CTLA-4 Ab-coated DC (anti-CTLA-4-Ab DC) showed significantly less proliferative response and enhanced IL-10 and TGF-beta1 production upon exposure to beta cell Ags. Furthermore, these mice showed increased frequency of Foxp3+ and IL-10+ T cells, less severe insulitis, and a significant delay in the onset of hyperglycemia compared with mice treated with control Ab-coated DCs. Further analyses showed that diabetogenic T cell function was modulated primarily through the induction of Foxp3 and IL-10 expression upon Ag presentation by anti-CTLA-4-Ab DCs. The induction of Foxp3 and IL-10 expression appeared to be a consequence of increased TGF-beta1 production by T cells activated using anti-CTLA-4-Ab DCs, and this effect could be enhanced by the addition of exogenous IL-2 or TGF-beta1. Collectively, this study demonstrates the potential of a DC-directed CTLA-4 engagement approach not only in treating autoimmunity in type 1 diabetes, but also in altering diabetogenic T cell function ex vivo for therapy.
Figures




Similar articles
-
Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) -adaptive regulatory T cell generation.J Immunol. 2008 May 15;180(10):6566-76. doi: 10.4049/jimmunol.180.10.6566. J Immunol. 2008. PMID: 18453575 Free PMC article.
-
Enhanced engagement of CTLA-4 induces antigen-specific CD4+CD25+Foxp3+ and CD4+CD25- TGF-beta 1+ adaptive regulatory T cells.J Immunol. 2007 Oct 15;179(8):5191-203. doi: 10.4049/jimmunol.179.8.5191. J Immunol. 2007. PMID: 17911604
-
Antigen-specific induced T regulatory cells impair dendritic cell function via an IL-10/MARCH1-dependent mechanism.J Immunol. 2013 Dec 15;191(12):5875-84. doi: 10.4049/jimmunol.1301693. Epub 2013 Nov 11. J Immunol. 2013. PMID: 24218453 Free PMC article.
-
Breaking T cell tolerance to beta cell antigens by merocytic dendritic cells.Cell Mol Life Sci. 2011 Sep;68(17):2873-83. doi: 10.1007/s00018-011-0730-6. Epub 2011 May 31. Cell Mol Life Sci. 2011. PMID: 21626409 Free PMC article. Review.
-
Immunoregulatory pathways controlling progression of autoimmunity in NOD mice.Ann N Y Acad Sci. 2008 Dec;1150:300-10. doi: 10.1196/annals.1447.046. Ann N Y Acad Sci. 2008. PMID: 19120317 Free PMC article. Review.
Cited by
-
Complex dietary polysaccharide modulates gut immune function and microbiota, and promotes protection from autoimmune diabetes.Immunology. 2019 May;157(1):70-85. doi: 10.1111/imm.13048. Epub 2019 Mar 7. Immunology. 2019. PMID: 30712258 Free PMC article.
-
CTLA-4⁺ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis.Cancer Res. 2015 Jun 1;75(11):2200-10. doi: 10.1158/0008-5472.CAN-14-2788. Epub 2015 Apr 1. Cancer Res. 2015. PMID: 25832655 Free PMC article.
-
A novel pancreatic β-cell targeting bispecific-antibody (BsAb) can prevent the development of type 1 diabetes in NOD mice.Clin Immunol. 2014 Jul;153(1):187-98. doi: 10.1016/j.clim.2014.04.014. Epub 2014 May 2. Clin Immunol. 2014. PMID: 24792135 Free PMC article.
-
Engineered Dendritic Cell-Directed Concurrent Activation of Multiple T cell Inhibitory Pathways Induces Robust Immune Tolerance.Sci Rep. 2019 Aug 19;9(1):12065. doi: 10.1038/s41598-019-48464-y. Sci Rep. 2019. PMID: 31427630 Free PMC article.
-
Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response.J Immunol. 2014 Oct 1;193(7):3308-21. doi: 10.4049/jimmunol.1400186. Epub 2014 Aug 20. J Immunol. 2014. PMID: 25143443 Free PMC article.
References
-
- Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. - PubMed
-
- Gambelunghe G, Brozzetti A, Ghaderi M, Candeloro P, Tortoioli C, Falorni A. MICA gene polymorphism in the pathogenesis of type 1 diabetes. Ann N Y Acad Sci. 2007;1110:92–98. - PubMed
-
- Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, Howlett S, Hunter K, Rainbow D, Rosa RL, Smink LJ, Todd JA, Peterson LB. Type 1 diabetes genes and pathways shared by humans and NOD mice. J. Autoimmun. 2005;25 Suppl:29–33. - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

