Invariant NKT cell development requires a full complement of functional CD3 zeta immunoreceptor tyrosine-based activation motifs
- PMID: 20483726
- PMCID: PMC2947369
- DOI: 10.4049/jimmunol.0902058
Invariant NKT cell development requires a full complement of functional CD3 zeta immunoreceptor tyrosine-based activation motifs
Abstract
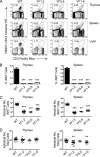
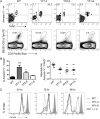
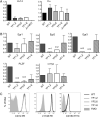
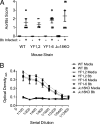
Invariant NKT (iNKT) cells regulate early immune responses to infections, in part because of their rapid release of IFN-gamma and IL-4. iNKT cells are proposed to reduce the severity of Lyme disease following Borrelia burgdorferi infection. Unlike conventional T cells, iNKT cells express an invariant alphabeta TCR that recognizes lipids bound to the MHC class I-like molecule, CD1d. Furthermore, these cells are positively selected following TCR interactions with glycolipid/CD1d complexes expressed on CD4+CD8+ thymocytes. Whereas conventional T cell development can proceed with as few as 4/10 CD3 immunoreceptor tyrosine-based activation motifs (ITAMs), little is known about the ITAM requirements for iNKT cell selection and expansion. We analyzed iNKT cell development in CD3 zeta transgenic lines with various tyrosine-to-phenylalanine substitutions (YF) that eliminated the functions of the first (YF1,2), third (YF5,6), or all three (YF1-6) CD3 zeta ITAMs. iNKT cell numbers were significantly reduced in the thymus, spleen, and liver of all YF mice compared with wild type mice. The reduced numbers of iNKT cells resulted from significant reductions in the expression of the early growth response 2 and promyelocytic leukemia zinc finger transcription factors. In the mice with few to no iNKT cells, there was no difference in the severity of Lyme arthritis compared with wild type controls, following infections with the spirochete B. burgdorferi. These findings indicate that a full complement of functional CD3 zeta ITAMs is required for effective iNKT cell development.
Figures


References
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. - PubMed
-
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007;5:405–417. - PubMed
-
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat. Rev. Immunol. 2004;4:231–237. - PubMed
-
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. - PubMed
-
- Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 2000;165:4797–4801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

