Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling
- PMID: 20483728
- PMCID: PMC2887731
- DOI: 10.4049/jimmunol.0904133
Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling
Abstract
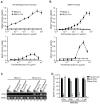
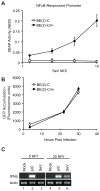
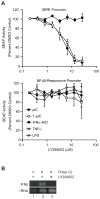
Innate immune pathways are early defense responses important for the immediate control and eventual clearance of many pathogens, where signaling is initiated via pattern recognition receptor (PRR)-mediated events that occur in a ligand- and cell-type specific manner. Within CNS neurons, innate immune pathways are likely crucial to control pathogens that target these essential yet virtually irreplaceable cells. However, relatively little is known about the induction and regulation of neuronal PRR signaling. In this report, we used human neuronal cell lines and primary rat neuronal cultures to examine PRR expression and function. We found that several innate immune receptor ligands, including Sendai virus, the dsRNA mimetic polyinosinic-polycytidylic acid, and LPS all activated differentiation-dependent neuronal innate immune pathways. Functional genetic analyses revealed that IFN regulatory factor 3-mediated pathways that resulted in IFN-beta transcriptional upregulation were activated in cultured human neuronal cells by the PRRs TLR3, MDA5, or RIG-I in a ligand-specific manner. Furthermore, genome-wide transcriptional array and targeted genetic and pharmacologic analyses identified PI3K signaling as crucial for the induction of innate immune pathways in neurons. These results indicate that human neuronal cells possess specific and functional PRR pathways essential for the effective induction of innate immune responses, and suggest that neurons can play an active role in defense against neurotropic pathogens.
Figures




Similar articles
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Combined CpG and poly I:C stimulation of monocytes results in unique signaling activation not observed with the individual ligands.Cell Signal. 2013 Nov;25(11):2246-54. doi: 10.1016/j.cellsig.2013.07.014. Epub 2013 Jul 19. Cell Signal. 2013. PMID: 23876795
-
TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk.J Dent Res. 2011 Apr;90(4):417-27. doi: 10.1177/0022034510381264. Epub 2010 Oct 12. J Dent Res. 2011. PMID: 20940366 Free PMC article. Review.
-
TLR and RLR Signaling Are Reprogrammed in Opposite Directions after Detection of Viral Infection.J Immunol. 2015 Nov 1;195(9):4387-95. doi: 10.4049/jimmunol.1500079. Epub 2015 Sep 21. J Immunol. 2015. PMID: 26392465
-
Class A Scavenger Receptor-Mediated Double-Stranded RNA Internalization Is Independent of Innate Antiviral Signaling and Does Not Require Phosphatidylinositol 3-Kinase Activity.J Immunol. 2015 Oct 15;195(8):3858-65. doi: 10.4049/jimmunol.1501028. Epub 2015 Sep 11. J Immunol. 2015. PMID: 26363049 Free PMC article.
Cited by
-
Understanding and altering cell tropism of vesicular stomatitis virus.Virus Res. 2013 Sep;176(1-2):16-32. doi: 10.1016/j.virusres.2013.06.003. Epub 2013 Jun 22. Virus Res. 2013. PMID: 23796410 Free PMC article. Review.
-
Host immune responses in the central nervous system during fungal infections.Immunol Rev. 2022 Oct;311(1):50-74. doi: 10.1111/imr.13101. Epub 2022 Jun 7. Immunol Rev. 2022. PMID: 35672656 Free PMC article. Review.
-
The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion.Brain Behav Immun. 2018 Nov;74:176-185. doi: 10.1016/j.bbi.2018.09.004. Epub 2018 Sep 5. Brain Behav Immun. 2018. PMID: 30195028 Free PMC article.
-
Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15625-30. doi: 10.1073/pnas.1005807107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713712 Free PMC article.
-
Immunomodulatory Strategies in Herpes Simplex Virus Encephalitis.Clin Microbiol Rev. 2020 Feb 12;33(2):e00105-19. doi: 10.1128/CMR.00105-19. Print 2020 Mar 18. Clin Microbiol Rev. 2020. PMID: 32051176 Free PMC article. Review.
References
-
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. - PubMed
-
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. - PubMed
-
- Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

