Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells
- PMID: 20483730
- PMCID: PMC2965829
- DOI: 10.4049/jimmunol.1000600
Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells
Abstract
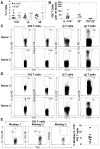
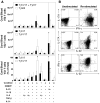
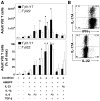
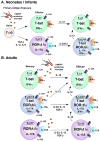
Human gammadelta T cells expressing the Vgamma2Vdelta2 TCR play important roles in immune responses to microbial pathogens by monitoring prenyl pyrophosphate isoprenoid metabolites. Most adult Vgamma2Vdelta2 cells are memory cytotoxic cells that produce IFN-gamma. Recently, murine gammadelta T cells were found to be major sources of IL-17A in antimicrobial and autoimmune responses. To determine if primate gammadelta T cells play similar roles, we characterized IL-17A and IL-22 production by Vgamma2Vdelta2 cells. IL-17A-producing memory Vgamma2Vdelta2 cells exist at low but significant frequencies in adult humans (1:2762 T cells) and at even higher frequencies in adult rhesus macaques. Higher levels of Vgamma2Vdelta2 cells produce IL-22 (1:1864 T cells), although few produce both IL-17A and IL-22. Unlike adult humans, in whom many IL-17A+ Vgamma2Vdelta2 cells also produce IFN-gamma (Tgammadelta1/17), the majority of adult macaques IL-17A+ Vdelta2 cells (Tgammadelta17) do not produce IFN-gamma. To define the cytokine requirements for Tgammadelta17 cells, we stimulated human neonatal Vgamma2Vdelta2 cells with the bacterial Ag, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate, and various cytokines and mAbs in vitro. We find that IL-6, IL-1beta, and TGF-beta are required to generate Tgammadelta17 cells in neonates, whereas Tgammadelta1/17 cells additionally required IL-23. In adults, memory Tgammadelta1/17 and Tgammadelta17 cells required IL-23, IL-1beta, and TGF-beta, but not IL-6. IL-22-producing cells showed similar requirements. Both neonatal and adult IL-17A+ Vgamma2Vdelta2 cells expressed elevated levels of retinoid-related orphan receptor gammat. Our data suggest that, like Th17 alphabeta T cells, Vgamma2Vdelta2 T cells can be polarized into Tgammadelta17 and Tgammadelta1/17 populations with distinct cytokine requirements for their initial polarization and later maintenance.
Conflict of interest statement
The authors have no financial conflict of interest
Figures



Similar articles
-
Regulation and function of IL-17A- and IL-22-producing γδ T cells.Cell Mol Life Sci. 2011 Jul;68(14):2371-90. doi: 10.1007/s00018-011-0700-z. Epub 2011 May 15. Cell Mol Life Sci. 2011. PMID: 21573786 Free PMC article. Review.
-
Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis.PLoS Pathog. 2010 Feb 26;6(2):e1000789. doi: 10.1371/journal.ppat.1000789. PLoS Pathog. 2010. PMID: 20195465 Free PMC article.
-
Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vγ2Vδ2 T cells after Mycobacterium tuberculosis infection or vaccination.Eur J Immunol. 2015 Feb;45(2):442-51. doi: 10.1002/eji.201444635. Eur J Immunol. 2015. PMID: 25141829 Free PMC article.
-
Gammadelta T cell immune manipulation during chronic phase of simian-human immunodeficiency virus infection [corrected] confers immunological benefits.J Immunol. 2009 Oct 15;183(8):5407-17. doi: 10.4049/jimmunol.0901760. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786533 Free PMC article.
-
Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens.Immunol Rev. 2007 Feb;215:59-76. doi: 10.1111/j.1600-065X.2006.00479.x. Immunol Rev. 2007. PMID: 17291279 Review.
Cited by
-
Mechanisms underlying lineage commitment and plasticity of human γδ T cells.Cell Mol Immunol. 2013 Jan;10(1):30-4. doi: 10.1038/cmi.2012.42. Epub 2012 Oct 22. Cell Mol Immunol. 2013. PMID: 23085943 Free PMC article. Review.
-
Plasticity of γδ T Cells: Impact on the Anti-Tumor Response.Front Immunol. 2014 Dec 8;5:622. doi: 10.3389/fimmu.2014.00622. eCollection 2014. Front Immunol. 2014. PMID: 25538706 Free PMC article. Review.
-
Antigen-presenting effects of effector memory Vγ9Vδ2 T cells in rheumatoid arthritis.Cell Mol Immunol. 2012 May;9(3):245-54. doi: 10.1038/cmi.2011.50. Epub 2011 Dec 5. Cell Mol Immunol. 2012. PMID: 22139198 Free PMC article.
-
The Jekyll and Hyde story of IL17-Producing γδT Cells.Front Immunol. 2015 Feb 4;6:37. doi: 10.3389/fimmu.2015.00037. eCollection 2015. Front Immunol. 2015. PMID: 25699053 Free PMC article. Review.
-
Key Features of Gamma-Delta T-Cell Subsets in Human Diseases and Their Immunotherapeutic Implications.Front Immunol. 2017 Jun 30;8:761. doi: 10.3389/fimmu.2017.00761. eCollection 2017. Front Immunol. 2017. PMID: 28713381 Free PMC article. Review.
References
-
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J Immunol. 1998;160:3513–3521. - PubMed
-
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. - PMC - PubMed
-
- Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. - PubMed
-
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

