Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine
- PMID: 20483731
- PMCID: PMC3145319
- DOI: 10.4049/jimmunol.0903116
Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine
Abstract
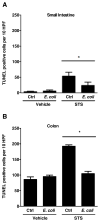
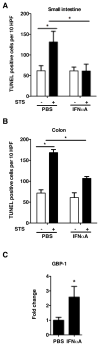
Appropriate microbial colonization protects the developing intestine by promoting epithelial barrier function and fostering mucosal tolerance to luminal bacteria. Commensal flora mediate their protective effects through TLR9-dependent activation of cytokines, such as type I IFNs (alpha, beta) and IL-10. Although IFN-beta promotes apoptosis, IFN-alpha activates specific antiapoptotic target genes whose actions preserve epithelial barrier integrity. We have recently identified guanylate binding protein-1 (GBP-1) as an antiapoptotic protein, regulated by both type I and type II IFNs, that promotes intestinal epithelial barrier integrity in mature intestine. However, the mechanisms by which commensal bacteria regulate epithelial apoptosis during colonization of immature intestine and the contributions of GBP-1 are unknown. The healthy newborn intestine is initially colonized with bacterial species present in the maternal gastrointestinal tract, including nonpathogenic Escherichia coli. Therefore, we examined the influence of commensal E. coli on cytokine expression and candidate mediators of apoptosis in preweaned mice. Specifically, enteral exposure of 2 wk-old mice to commensal E. coli for 24 h selectively increased both IFN-alphaA and GBP-1 mRNA expression and prevented staurosporine-induced epithelial apoptosis. Exogenous IFN-alphaA treatment also induced GBP-1 expression and protected against staurosporine-induced apoptosis in a GBP-1 dependent manner, both in vitro and ex vivo. These findings identify a role for IFN-alphaA-mediated GBP-1 expression in the prevention of intestinal epithelial apoptosis by commensal bacteria. Thus IFN-alphaA mediates the beneficial effects of commensal bacteria and may be a promising therapeutic target to promote barrier integrity and prevent the inappropriate inflammatory responses seen in developing intestine as in necrotizing enterocolitis.
Figures





Similar articles
-
Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut.Gut. 2005 May;54(5):623-9. doi: 10.1136/gut.2004.056028. Gut. 2005. PMID: 15831905 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis.Mucosal Immunol. 2009 Jan;2(1):33-42. doi: 10.1038/mi.2008.62. Epub 2008 Sep 17. Mucosal Immunol. 2009. PMID: 19079332 Free PMC article.
-
Escherichia coli K12 Upregulates Programmed Cell Death Ligand 1 (PD-L1) Expression in Gamma Interferon-Sensitized Intestinal Epithelial Cells via the NF-κB Pathway.Infect Immun. 2020 Dec 15;89(1):e00618-20. doi: 10.1128/IAI.00618-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33046511 Free PMC article.
-
Pathophysiological role of guanylate-binding proteins in gastrointestinal diseases.World J Gastroenterol. 2016 Jul 28;22(28):6434-43. doi: 10.3748/wjg.v22.i28.6434. World J Gastroenterol. 2016. PMID: 27605879 Free PMC article. Review.
Cited by
-
The guanylate-binding proteins: emerging insights into the biochemical properties and functions of this family of large interferon-induced guanosine triphosphatase.J Interferon Cytokine Res. 2011 Jan;31(1):89-97. doi: 10.1089/jir.2010.0102. Epub 2010 Dec 13. J Interferon Cytokine Res. 2011. PMID: 21142871 Free PMC article. Review.
-
Human breast milk and the gastrointestinal innate immune system.Clin Perinatol. 2014 Jun;41(2):423-35. doi: 10.1016/j.clp.2014.02.011. Clin Perinatol. 2014. PMID: 24873841 Free PMC article. Review.
-
Deficiency in the 15 kDa selenoprotein inhibits human colon cancer cell growth.Nutrients. 2011 Sep;3(9):805-17. doi: 10.3390/nu3090805. Epub 2011 Sep 5. Nutrients. 2011. PMID: 22254125 Free PMC article.
-
Gut Barrier Damage and Gut Translocation of Pathogen Molecules in Lupus, an Impact of Innate Immunity (Macrophages and Neutrophils) in Autoimmune Disease.Int J Mol Sci. 2022 Jul 26;23(15):8223. doi: 10.3390/ijms23158223. Int J Mol Sci. 2022. PMID: 35897790 Free PMC article. Review.
-
A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis.Cell Res. 2023 May;33(5):372-388. doi: 10.1038/s41422-023-00790-7. Epub 2023 Apr 13. Cell Res. 2023. PMID: 37055591 Free PMC article.
References
-
- Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. - PubMed
-
- Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 2005;175:4194–4198. - PubMed
-
- Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006;1072:351–355. - PubMed
-
- Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

