Differential roles of IL-2-inducible T cell kinase-mediated TCR signals in tissue-specific localization and maintenance of skin intraepithelial T cells
- PMID: 20483745
- PMCID: PMC2941197
- DOI: 10.4049/jimmunol.1000453
Differential roles of IL-2-inducible T cell kinase-mediated TCR signals in tissue-specific localization and maintenance of skin intraepithelial T cells
Abstract
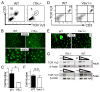
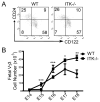
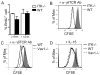
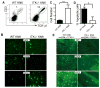
Tissue-specific innate-like gammadelta T cells are important components of the immune system critical for the first line of defense, but mechanisms underlying their tissue-specific development are poorly understood. Our study with prototypical skin-specific intraepithelial gammadeltaT lymphocytes (sIELs) found that among different thymic gammadelta T cell subsets fetal thymic precursors of sIELs specifically acquire a unique skin-homing property after positive selection, suggesting an important role of the TCR selection signaling in "programming" them for tissue-specific development. In this study, we identified IL-2-inducible T cell kinase (ITK) as a critical signal molecule regulating the acquirement of the skin-homing property by the fetal thymic sIEL precursors. In ITK knockout mice, the sIEL precursors could not undergo positive selection-associated upregulation of thymus-exiting and skin-homing molecules sphingosine-1-phosphate receptor 1 and CCR10 and accumulated in the thymus. However, the survival and expansion of sIELs in the skin did not require ITK-transduced TCR signaling, whereas its persistent activation impaired sIEL development by inducing apoptosis. These findings provide insights into molecular mechanisms underlying differential requirements of TCR signaling in peripheral localization and maintenance of the tissue-specific T cells.
Figures

References
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annual review of immunology. 2000;18:975–1026. - PubMed
-
- Raulet DH, Spencer DM, Hsiang YH, Goldman JP, Bix M, Liao NS, Zijstra M, Jaenisch R, Correa I. Control of gamma delta T-cell development. Immunological reviews. 1991;120:185–204. - PubMed
-
- Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells. Phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period. J Immunol. 1989;143:2431–2438. - PubMed
-
- Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. - PubMed
-
- Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal gammadelta cells with disrupted primary Vgamma gene usage. Science (New York, NY) 1998;279:1729–1733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

