Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis
- PMID: 20483789
- PMCID: PMC2921223
- DOI: 10.4049/jimmunol.0902009
Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis
Abstract
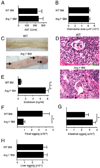
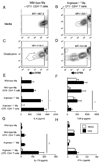
Alternatively activated macrophages prevent lethal intestinal pathology caused by worm ova in mice infected with the human parasite Schistosoma mansoni through mechanisms that are currently unclear. This study demonstrates that arginase I (Arg I), a major product of IL-4- and IL-13-induced alternatively activated macrophages, prevents cachexia, neutrophilia, and endotoxemia during acute schistosomiasis. Specifically, Arg I-positive macrophages promote TGF-beta production and Foxp3 expression, suppress Ag-specific T cell proliferation, and limit Th17 differentiation. S. mansoni-infected Arg I-deficient bone marrow chimeras develop a marked accumulation of worm ova within the ileum but impaired fecal egg excretion compared with infected wild-type bone marrow chimeras. Worm ova accumulation in the intestines of Arg I-deficient bone marrow chimeras was associated with intestinal hemorrhage and production of molecules associated with classical macrophage activation (increased production of IL-6, NO, and IL-12/IL-23p40), but whereas inhibition of NO synthase-2 has marginal effects, IL-12/IL-23p40 neutralization abrogates both cachexia and intestinal inflammation and reduces the number of ova within the gut. Thus, macrophage-derived Arg I protects hosts against excessive tissue injury caused by worm eggs during acute schistosomiasis by suppressing IL-12/IL-23p40 production and maintaining the Treg/Th17 balance within the intestinal mucosa.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






References
-
- Urban JF, Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J. Immunol. 2001;167:6078–6081. - PubMed
-
- Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat. Rev. Microbiol. 2004;2:12–13. - PubMed
-
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosemiasis. Lancet. 2006;368:1106–1118. - PubMed
-
- La Flamme AC, Patton EA, Bauman B, Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 2001;166:1903–1911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

