Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma
- PMID: 20483988
- PMCID: PMC2890814
- DOI: 10.1073/pnas.1005383107
Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma
Abstract
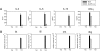
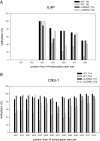
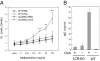
Previous studies have shown that Th2 cytokine genes on mouse chromosome 11 are coordinately regulated by the Th2 locus control region (LCR). To examine the in vivo function of Th2 LCR, we generated CD4-specific Th2 LCR-deficient (cLCR KO) mice using Cre-LoxP recombination. The number of CD4 T cells in the cLCR KO mouse was comparable to that in wild-type mice. The expression of Th2 cytokines was dramatically reduced in in vitro-stimulated naïve CD4 T cells. Deletion of the LCR led to a loss of general histone H3 acetylation and histone H3-K4 methylation, and demethylation of DNA in the Th2 cytokine locus. Upon ovalbumin challenge in the mouse model of allergic asthma, cLCR KO mice exhibited marked reduction in the recruitment of eosinophils and lymphocytes in the bronchoalveolar lavage fluid, serum IgE level, lung airway inflammation, mucus production in the airway walls, and airway hyperresponsiveness. These results directly demonstrate that the Th2 LCR is critically important in the regulation of Th2 cytokine genes, in chromatin remodeling of the Th2 cytokine locus, and in the pathogenesis of allergic asthma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
RHS6 coordinately regulates the Th2 cytokine genes by recruiting GATA3, SATB1, and IRF4.Allergy. 2017 May;72(5):772-782. doi: 10.1111/all.13078. Epub 2016 Nov 23. Allergy. 2017. PMID: 27878828
-
Increased Th2 cytokine secretion, eosinophilic airway inflammation, and airway hyperresponsiveness in neurturin-deficient mice.J Immunol. 2011 Jun 1;186(11):6497-504. doi: 10.4049/jimmunol.1001673. Epub 2011 Apr 20. J Immunol. 2011. PMID: 21508262
-
Hypersensitive site 6 of the Th2 locus control region is essential for Th2 cytokine expression.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):6955-60. doi: 10.1073/pnas.1304720110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569250 Free PMC article.
-
New insights into the role of cytokines in asthma.J Clin Pathol. 2001 Aug;54(8):577-89. doi: 10.1136/jcp.54.8.577. J Clin Pathol. 2001. PMID: 11477111 Free PMC article. Review.
-
Orchestration of epithelial-derived cytokines and innate immune cells in allergic airway inflammation.Cytokine Growth Factor Rev. 2018 Feb;39:19-25. doi: 10.1016/j.cytogfr.2017.11.004. Epub 2017 Nov 21. Cytokine Growth Factor Rev. 2018. PMID: 29169815 Free PMC article. Review.
Cited by
-
Analysis of lncRNA Expression in Patients With Eosinophilic and Neutrophilic Asthma Focusing on LNC_000127.Front Genet. 2019 Mar 19;10:141. doi: 10.3389/fgene.2019.00141. eCollection 2019. Front Genet. 2019. PMID: 30941157 Free PMC article.
-
Epigenetic regulation of immune function in asthma.J Allergy Clin Immunol. 2022 Aug;150(2):259-265. doi: 10.1016/j.jaci.2022.06.002. Epub 2022 Jun 16. J Allergy Clin Immunol. 2022. PMID: 35717251 Free PMC article. Review.
-
TL1A priming induces a multi-cytokine Th9 cell phenotype that promotes robust allergic inflammation in murine models of asthma.Mucosal Immunol. 2024 Aug;17(4):537-553. doi: 10.1016/j.mucimm.2024.03.006. Epub 2024 Mar 15. Mucosal Immunol. 2024. PMID: 38493956 Free PMC article.
-
Aberrant Expression of TH2LCRR and GATA3 in Peripheral Blood Mononuclear Cells of Patients with Acute-Phase Schizophrenia: Integrative Bioinformatics Analysis and Experimental Study.Mol Neurobiol. 2025 May 23. doi: 10.1007/s12035-025-05060-8. Online ahead of print. Mol Neurobiol. 2025. PMID: 40408026
-
Epigenetic mechanisms and the development of asthma.J Allergy Clin Immunol. 2012 Dec;130(6):1243-55. doi: 10.1016/j.jaci.2012.07.052. Epub 2012 Sep 29. J Allergy Clin Immunol. 2012. PMID: 23026498 Free PMC article. Review.
References
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
-
- Dong C, Flavell RA. Th1 and Th2 cells. Curr Opin Hematol. 2001;8:47–51. - PubMed
-
- Glimcher LH, Murphy KM. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. - PubMed
-
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. - PubMed
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

