Vinculin is a dually regulated actin filament barbed end-capping and side-binding protein
- PMID: 20484056
- PMCID: PMC2906333
- DOI: 10.1074/jbc.M110.102830
Vinculin is a dually regulated actin filament barbed end-capping and side-binding protein
Abstract
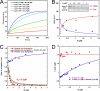
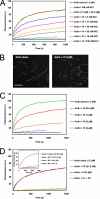
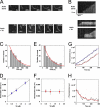
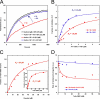
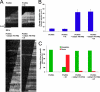
The focal adhesion protein vinculin is an actin-binding protein involved in the mechanical coupling between the actin cytoskeleton and the extracellular matrix. An autoinhibitory interaction between the N-terminal head (Vh) and the C-terminal tail (Vt) of vinculin masks an actin filament side-binding domain in Vt. The binding of several proteins to Vh disrupts this intramolecular interaction and exposes the actin filament side-binding domain. Here, by combining kinetic assays and microscopy observations, we show that Vt inhibits actin polymerization by blocking the barbed ends of actin filaments. In low salt conditions, Vt nucleates actin filaments capped at their barbed ends. We determined that the interaction between vinculin and the barbed end is characterized by slow association and dissociation rate constants. This barbed end capping activity requires C-terminal amino acids of Vt that are dispensable for actin filament side binding. Like the side-binding domain, the capping domain of vinculin is masked by an autoinhibitory interaction between Vh and Vt. In contrast to the side-binding domain, the capping domain is not unmasked by the binding of a talin domain to Vh and requires the dissociation of an additional autoinhibitory interaction. Finally, we show that vinculin and the formin mDia1, which is involved in the processive elongation of actin filaments in focal adhesions, compete for actin filament barbed ends.
Figures

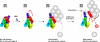
References
-
- Ziegler W. H., Liddington R. C., Critchley D. R. (2006) Trends Cell Biol. 16, 453–460 - PubMed
-
- Zamir E., Geiger B. (2001) J. Cell Sci. 114, 3583–3590 - PubMed
-
- Volberg T., Geiger B., Kam Z., Pankov R., Simcha I., Sabanay H., Coll J. L., Adamson E., Ben-Ze'ev A. (1995) J. Cell Sci. 108, 2253–2260 - PubMed
-
- Xu W., Coll J. L., Adamson E. D. (1998) J. Cell Sci. 111, 1535–1544 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

