The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial
- PMID: 20484090
- PMCID: PMC4165390
- DOI: 10.1177/0272989X10369007
The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial
Abstract
Background: Eliciting patients' preferences within a framework of shared decision making (SDM) has been advocated as a strategy for increasing colorectal cancer (CRC) screening adherence. Our objective was to assess the effectiveness of a novel decision aid on SDM in the primary care setting.
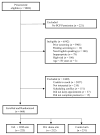
Methods: An interactive, computer-based decision aid for CRC screening was developed and evaluated within the context of a randomized controlled trial. A total of 665 average-risk patients (mean age, 57 years; 60% female; 63% black, 6% Hispanic) were allocated to 1 of 2 intervention arms (decision aid alone, decision aid plus personalized risk assessment) or a control arm. The interventions were delivered just prior to a scheduled primary care visit. Outcome measures (patient preferences, knowledge, satisfaction with the decision-making process [SDMP], concordance between patient preference and test ordered, and intentions) were evaluated using prestudy/poststudy visit questionnaires and electronic scheduling.
Results: Overall, 95% of patients in the intervention arms identified a preferred screening option based on values placed on individual test features. Mean cumulative knowledge, SDMP, and intention scores were significantly higher for both intervention groups compared with the control group. Concordance between patient preference and test ordered was 59%. Patients who preferred colonoscopy were more likely to have a test ordered than those who preferred an alternative option (83% v. 70%; P < 0.01). Intention scores were significantly higher when the test ordered reflected patient preferences.
Conclusions: Our interactive computer-based decision aid facilitates SDM, but overall effectiveness is determined by the extent to which providers comply with patient preferences.
Figures

References
-
- Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. - PubMed
-
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous Polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. - PubMed
-
- US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. - PubMed
-
- Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–30. - PubMed
-
- Leard LE, Savides TJ, Ganiats TG. Patient preferences for colorectal cancer screening. J Fam Pract. 1997;45:211–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

