Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer
- PMID: 20484123
- PMCID: PMC3061828
- DOI: 10.1634/theoncologist.2010-0029
Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer
Abstract
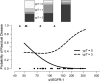
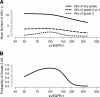
We explored plasma and urinary concentrations of two members of the vascular endothelial growth factor (VEGF) family and their receptors as potential response and toxicity biomarkers of bevacizumab with neoadjuvant chemoradiation in patients with localized rectal cancer. The concentrations of VEGF, placental growth factor (PlGF), soluble VEGF receptor 1 (sVEGFR-1), and sVEGFR-2 were measured in plasma and urine at baseline and during treatment. Pretreatment values and changes over time were analyzed as potential biomarkers of pathological response to treatment as well as for acute toxicity in patients with locally advanced rectal cancer treated prospectively in 2002-2008 with neoadjuvant bevacizumab, 5-fluorouracil, radiation therapy, and surgery in a phase I/II trial. Of all biomarkers, pretreatment plasma sVEGFR-1-an endogenous blocker of VEGF and PlGF, and a factor linked with "vascular normalization"-was associated with both primary tumor regression and the development of adverse events after neoadjuvant bevacizumab and chemoradiation. Based on the findings in this exploratory study, we propose that plasma sVEGFR-1 should be further studied as a potential biomarker to stratify patients in future studies of bevacizumab and/or cytotoxics in the neoadjuvant setting.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
References
-
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

