cAMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. Implication in mitogenesis and activation of CDK4
- PMID: 20484410
- PMCID: PMC2903905
- DOI: 10.1210/me.2010-0087
cAMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. Implication in mitogenesis and activation of CDK4
Abstract
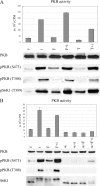
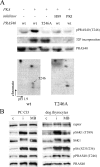
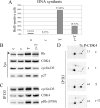
How cAMP-dependent protein kinases [protein kinase A (PKA)] transduce the mitogenic stimulus elicited by TSH in thyroid cells to late activation of cyclin D3-cyclin-dependent kinase 4 (CDK4) remains enigmatic. Here we show in PC Cl3 rat thyroid cells that TSH/cAMP, like insulin, activates the mammalian target of rapamycin (mTOR)-raptor complex (mTORC1) leading to phosphorylation of S6K1 and 4E-BP1. mTORC1-dependent S6K1 phosphorylation in response to both insulin and cAMP required amino acids, whereas inhibition of AMP-activated protein kinase and glycogen synthase kinase 3 enhanced insulin but not cAMP effects. Unlike insulin, TSH/cAMP did not activate protein kinase B or induce tuberous sclerosis complex 2 phosphorylation at T1462 and Y1571. However, like insulin, TSH/cAMP produced a stable increase in mTORC1 kinase activity that was associated with augmented 4E-BP1 binding to raptor. This could be caused in part by T246 phosphorylation of PRAS40, which was found as an in vitro substrate of PKA. Both in PC Cl3 cells and primary dog thyrocytes, rapamycin inhibited DNA synthesis and retinoblastoma protein phosphorylation induced by TSH and insulin. Although rapamycin reduced cyclin D3 accumulation, the abundance of cyclin D3-CDK4 complexes was not affected. However, rapamycin inhibited the activity of these complexes by decreasing the TSH and insulin-mediated stimulation of activating T172 phosphorylation of CDK4. We propose that mTORC1 activation by TSH, at least in part through PKA-dependent phosphorylation of PRAS40, crucially contributes to mediate cAMP-dependent mitogenesis by regulating CDK4 T172-phosphorylation.
Figures




Similar articles
-
Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland.J Biol Chem. 2003 Jun 13;278(24):21960-71. doi: 10.1074/jbc.M300805200. Epub 2003 Mar 30. J Biol Chem. 2003. PMID: 12668683
-
Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1).Mol Biol Cell. 2000 Mar;11(3):1061-76. doi: 10.1091/mbc.11.3.1061. Mol Biol Cell. 2000. PMID: 10712520 Free PMC article.
-
A requirement for cyclin D3-cyclin-dependent kinase (cdk)-4 assembly in the cyclic adenosine monophosphate-dependent proliferation of thyrocytes.J Cell Biol. 1998 Mar 23;140(6):1427-39. doi: 10.1083/jcb.140.6.1427. J Cell Biol. 1998. PMID: 9508775 Free PMC article.
-
The cyclin D3-CDK4-p27kip1 holoenzyme in thyroid epithelial cells: activation by TSH, inhibition by TGFbeta, and phosphorylations of its subunits demonstrated by two-dimensional gel electrophoresis.Exp Cell Res. 2003 Nov 15;291(1):135-49. doi: 10.1016/s0014-4827(03)00392-6. Exp Cell Res. 2003. PMID: 14597415
-
Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation.Colomb Med (Cali). 2012 Sep 30;43(3):235-43. eCollection 2012 Jul. Colomb Med (Cali). 2012. PMID: 24893199 Free PMC article. Review.
Cited by
-
Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma.Nat Rev Urol. 2022 May;19(5):305-320. doi: 10.1038/s41585-022-00571-8. Epub 2022 Mar 9. Nat Rev Urol. 2022. PMID: 35264774 Free PMC article. Review.
-
Crosstalk between beta-adrenergic and insulin signaling mediates mechanistic target of rapamycin hyperactivation in liver of high-fat diet-fed male mice.Physiol Rep. 2021 Jul;9(13):e14958. doi: 10.14814/phy2.14958. Physiol Rep. 2021. PMID: 34231324 Free PMC article.
-
CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point.PLoS Genet. 2013 May;9(5):e1003546. doi: 10.1371/journal.pgen.1003546. Epub 2013 May 30. PLoS Genet. 2013. PMID: 23737759 Free PMC article.
-
Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation.Mol Endocrinol. 2014 May;28(5):681-95. doi: 10.1210/me.2013-1377. Epub 2014 Mar 19. Mol Endocrinol. 2014. PMID: 24645679 Free PMC article.
-
cAMP inhibits mammalian target of rapamycin complex-1 and -2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity.Cell Signal. 2011 Dec;23(12):1927-35. doi: 10.1016/j.cellsig.2011.06.025. Epub 2011 Jul 6. Cell Signal. 2011. PMID: 21763421 Free PMC article.
References
-
- Dumont JE, Maenhaut C, Christophe D, Vassart G, Roger PP 2005 Thyroid regulatory factors. In: DeGroot LJ, Jameson JL, eds. Endocrinology. New York: Elsevier Saunders; 1837–1860
-
- Roger PP, Servais P, Dumont JE 1983 Stimulation by thyrotropin and cyclic AMP of the proliferation of quiescent canine thyroid cells cultured in a defined medium containing insulin. FEBS Lett 157:323–329 - PubMed
-
- Roger P, Taton M, Van Sande J, Dumont JE 1988 Mitogenic effects of thyrotropin and adenosine 3′,5′-monophosphate in differentiated normal human thyroid cells in vitro. J Clin Endocrinol Metab 66:1158–1165 - PubMed
-
- Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP 2001 Regulation of thyroid cell proliferation by thyrotropin and other factors : a critical evaluation of in vitro models. Endocr Rev 22:631–656 - PubMed
-
- Furuya F, Lu C, Willingham MC, Cheng SY 2007 Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 28:2451–2458 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

