Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma
- PMID: 20484421
- PMCID: PMC2892806
- DOI: 10.2967/jnumed.109.071290
Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma
Abstract
Panitumumab, a human monoclonal antibody that binds to the epidermal growth factor receptor (HER1), was approved by the Food and Drug Administration in 2006 for the treatment of patients with HER1-expressing carcinoma. In this article, we describe the preclinical development of (86)Y-CHX-A''-diethylenetriaminepentaacetic acid (DTPA)-panitumumab for quantitative PET of HER1-expressing carcinoma. Panitumumab was conjugated to CHX-A''-DTPA and radiolabeled with (86)Y. In vivo biodistribution, PET, blood clearance, area under the curve, area under the moment curve, and mean residence time were determined for mice bearing HER1-expressing human colorectal (LS-174T), prostate (PC-3), and epidermoid (A431) tumor xenografts. Receptor specificity was demonstrated by coinjection of 0.1 mg of panitumumab with the radioimmunoconjugate.
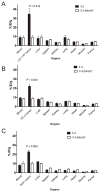
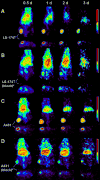
Results: (86)Y-CHX-A''-DTPA-panitumumab was routinely prepared with a specific activity exceeding 2 GBq/mg. Biodistribution and PET studies demonstrated a high HER1-specific tumor uptake of the radioimmunoconjugate. In mice bearing LS-174T, PC-3, or A431 tumors, the tumor uptake at 3 d was 34.6 +/- 5.9, 22.1 +/- 1.9, and 22.7 +/- 1.7 percentage injected dose per gram (%ID/g), respectively. The corresponding tumor uptake in mice coinjected with 0.1 mg of panitumumab was 9.3 +/- 1.5, 8.8 +/- 0.9, and 10.0 +/- 1.3 %ID/g, respectively, at the same time point, demonstrating specific blockage of the receptor. Normal organ and tumor uptake quantified by PET was closely related (r(2) = 0.95) to values determined by biodistribution studies. The LS-174T tumor had the highest area under the curve (96.8 +/- 5.6 %ID d g(-1)) and area under the moment curve (262.5 +/- 14.9 %ID d(2) g(-1)); however, the tumor mean residence times were identical for all 3 tumors (2.7-2.8 d).
Conclusion: This study demonstrates the potential of (86)Y-CHX-A''-DTPA-panitumumab for quantitative noninvasive PET of HER1-expressing tumors and represents the first step toward clinical translation.
Conflict of interest statement
Authors declare no conflict of interests.
Figures


Similar articles
-
PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab.J Nucl Med. 2012 Jan;53(1):113-20. doi: 10.2967/jnumed.111.094169. J Nucl Med. 2012. PMID: 22213822 Free PMC article.
-
PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A''-DTPA-cetuximab.Eur J Nucl Med Mol Imaging. 2010 Jul;37(7):1368-76. doi: 10.1007/s00259-009-1370-z. Epub 2010 Feb 13. Eur J Nucl Med Mol Imaging. 2010. PMID: 20155263 Free PMC article.
-
PET imaging of tumor angiogenesis in mice with VEGF-A-targeted (86)Y-CHX-A″-DTPA-bevacizumab.Int J Cancer. 2011 Feb 15;128(4):920-6. doi: 10.1002/ijc.25409. Int J Cancer. 2011. PMID: 20473899 Free PMC article.
-
86Y-CHX-A''-diethylenetriamine pentaacetic acid-panitumumab.2011 Aug 25 [updated 2011 Dec 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Aug 25 [updated 2011 Dec 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22171389 Free Books & Documents. Review.
-
111In/86Y-Labeled F(ab')2 fragment of panitumumab, a fully human monoclonal antibody directed against the extracellular domain III of the epidermal growth factor receptor.2012 Apr 30 [updated 2012 Jun 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Apr 30 [updated 2012 Jun 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22741182 Free Books & Documents. Review.
Cited by
-
Imaging using radiolabelled targeted proteins: radioimmunodetection and beyond.EJNMMI Radiopharm Chem. 2020 Jun 23;5(1):16. doi: 10.1186/s41181-020-00094-w. EJNMMI Radiopharm Chem. 2020. PMID: 32577943 Free PMC article. Review.
-
Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy.Pharmaceuticals (Basel). 2014 Mar 5;7(3):311-38. doi: 10.3390/ph7030311. Pharmaceuticals (Basel). 2014. PMID: 24603603 Free PMC article.
-
PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab.J Nucl Med. 2012 Jan;53(1):113-20. doi: 10.2967/jnumed.111.094169. J Nucl Med. 2012. PMID: 22213822 Free PMC article.
-
Synthesis and biological evaluation of panitumumab-IRDye800 conjugate as a fluorescence imaging probe for EGFR-expressing cancers.Medchemcomm. 2014 Sep;5(9):1337-1346. doi: 10.1039/C4MD00116H. Medchemcomm. 2014. PMID: 25431648 Free PMC article.
-
The use of radiocobalt as a label improves imaging of EGFR using DOTA-conjugated Affibody molecule.Sci Rep. 2017 Jul 20;7(1):5961. doi: 10.1038/s41598-017-05700-7. Sci Rep. 2017. PMID: 28729680 Free PMC article.
References
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. - PubMed
-
- Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26(5):263–274. - PubMed
-
- Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11(4):689–708. - PubMed
-
- Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33(4):369–385. - PubMed
-
- Patel DD, Goldberg RM. Cetuximab-associated infusion reactions: pathology and management. Oncology (Williston Park) 2006;20(11):1373–1382. discussion 1382, 1392–1374, 1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
