Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling
- PMID: 20484484
- PMCID: PMC2913031
- DOI: 10.1210/jc.2009-2696
Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling
Abstract
Objective: Our objective was to determine whether endothelial-dependent vasodilation is an essential mechanism by which insulin stimulates human skeletal muscle protein synthesis and anabolism.
Subjects: Subjects were healthy young adults (n=14) aged 31+/-2 yr.
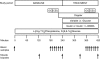
Design: Subjects were studied at baseline and during local leg infusion of insulin alone (control, n=7) or insulin plus the nitric oxide synthase inhibitor NG-monomethyl-L-arginine (L-NMMA, n=7) to prevent insulin-induced vasodilation.
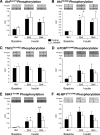
Methods: We measured skeletal muscle protein metabolism with stable isotope tracers, blood flow with indocyanine green, capillary recruitment with contrast enhanced ultrasound, glucose metabolism with stable isotope tracers, and phosphorylation of proteins associated with insulin (Akt) and amino acid-induced mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling (mTOR, S6 kinase 1, and eukaryotic initiation factor 4E-binding protein 1) with Western blot analysis.
Results: No basal differences between groups were detected. During insulin infusion, blood flow and capillary recruitment increased in the control (P<0.05) group only; Akt phosphorylation and glucose uptake increased in both groups (P<0.05), with no group differences; and mTORC1 signaling increased more in control (P<0.05) than in L-NMMA. Phenylalanine net balance increased (P<0.05) in both groups, but with opposite mechanisms: increased protein synthesis (basal, 0.051+/-0.006 %/h; insulin, 0.077+/-0.008 %/h; P<0.05) with no change in proteolysis in control and decreased proteolysis (P<0.05) with no change in synthesis (basal, 0.061+/-0.004 %/h; insulin, 0.050+/-0.006 %/h; P value not significant) in L-NMMA.
Conclusions: Endothelial-dependent vasodilation and the consequent increase in nutritive flow and mTORC1 signaling, rather than Akt signaling, are fundamental mechanisms by which insulin stimulates muscle protein synthesis in humans. Additionally, these data underscore that insulin modulates skeletal muscle proteolysis according to its effects on nutritive flow.
Figures


Similar articles
-
Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults.Diabetes. 2010 Nov;59(11):2764-71. doi: 10.2337/db10-0415. Epub 2010 Aug 19. Diabetes. 2010. PMID: 20724580 Free PMC article.
-
Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated.Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1597-605. doi: 10.1152/ajpendo.00307.2007. Epub 2007 Sep 18. Am J Physiol Endocrinol Metab. 2007. PMID: 17878222 Free PMC article.
-
Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis.J Physiol. 2009 Apr 1;587(Pt 7):1535-46. doi: 10.1113/jphysiol.2008.163816. Epub 2009 Feb 2. J Physiol. 2009. PMID: 19188252 Free PMC article. Clinical Trial.
-
With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging.Cell Metab. 2010 Jun 9;11(6):453-65. doi: 10.1016/j.cmet.2010.05.001. Cell Metab. 2010. PMID: 20519118 Free PMC article. Review.
-
Anabolic and catabolic pathways regulating skeletal muscle mass.Curr Opin Clin Nutr Metab Care. 2010 May;13(3):230-5. doi: 10.1097/MCO.0b013e32833781b5. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20154608 Free PMC article. Review.
Cited by
-
Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women.Exp Gerontol. 2015 May;65:1-7. doi: 10.1016/j.exger.2015.02.015. Epub 2015 Feb 28. Exp Gerontol. 2015. PMID: 25735236 Free PMC article.
-
Microbial translocation and skeletal muscle in young and old vervet monkeys.Age (Dordr). 2016 Jun;38(3):58. doi: 10.1007/s11357-016-9924-z. Epub 2016 May 18. Age (Dordr). 2016. PMID: 27194407 Free PMC article.
-
A Narrative Review of Non-Pharmacological Strategies for Managing Sarcopenia in Older Adults with Cardiovascular and Metabolic Diseases.Biology (Basel). 2023 Jun 21;12(7):892. doi: 10.3390/biology12070892. Biology (Basel). 2023. PMID: 37508325 Free PMC article. Review.
-
Insulin increases mRNA abundance of the amino acid transporter SLC7A5/LAT1 via an mTORC1-dependent mechanism in skeletal muscle cells.Physiol Rep. 2014 Mar 20;2(3):e00238. doi: 10.1002/phy2.238. Print 2014. Physiol Rep. 2014. PMID: 24760501 Free PMC article.
-
Ingestion of a variety of non-animal-derived dietary protein sources results in diverse postprandial plasma amino acid responses which differ between young and older adults.Br J Nutr. 2024 May 14;131(9):1540-1553. doi: 10.1017/S0007114524000163. Epub 2024 Jan 15. Br J Nutr. 2024. PMID: 38220222 Free PMC article. Clinical Trial.
References
-
- Kimball SR, Jefferson LS, Fadden P, Haystead TA, Lawrence Jr JC 1996 Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am J Physiol 270(2 Pt 1):C705–C709 - PubMed
-
- Kimball SR, Jurasinski CV, Lawrence Jr JC, Jefferson LS 1997 Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol 272(2 Pt 1):C754–C759 - PubMed
-
- O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA 2003 Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284:E110–E119 - PubMed
-
- Bennet WM, Rennie MJ 1991 Protein anabolic actions of insulin in the human body. Diabet Med 8:199–207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

