Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model
- PMID: 20484500
- PMCID: PMC2897626
- DOI: 10.1128/JVI.02444-09
Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model
Abstract
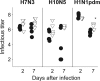
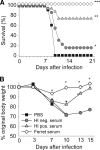
Our ability to rapidly respond to an emerging influenza pandemic is hampered somewhat by the lack of a susceptible small-animal model. To develop a more sensitive model, we pathotyped 18 low-pathogenic non-mouse-adapted influenza A viruses of human and avian origin in DBA/2 and C57BL/6 mice. The majority of the isolates (13/18) induced severe morbidity and mortality in DBA/2 mice upon intranasal challenge with 1 million infectious doses. Also, at a 100-fold-lower dose, more than 50% of the viruses induced severe weight loss, and mice succumbed to the infection. In contrast, only two virus strains were pathogenic for C57BL/6 mice upon high-dose inoculation. Therefore, DBA/2 mice are a suitable model to validate influenza A virus vaccines and antiviral therapies without the need for extensive viral adaptation. Correspondingly, we used the DBA/2 model to assess the level of protection afforded by preexisting pandemic H1N1 2009 virus (H1N1pdm) cross-reactive human antibodies detected by a hemagglutination inhibition assay. Passive transfer of these antibodies prior to infection protected mice from H1N1pdm-induced pathogenicity, demonstrating the effectiveness of these cross-reactive neutralizing antibodies in vivo.
Figures
References
-
- Centers for Disease Control and Prevention. 2009. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 58:521-524. - PubMed
-
- Chen, Z., S. Kadowaki, Y. Hagiwara, T. Yoshikawa, K. Matsuo, T. Kurata, and S. Tamura. 2000. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18:3214-3222. - PubMed
-
- Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. - PubMed
-
- de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, d. Q. Ha, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

