Phosphatidylinositol 3-kinase-, actin-, and microtubule-dependent transport of Semliki Forest Virus replication complexes from the plasma membrane to modified lysosomes
- PMID: 20484502
- PMCID: PMC2897599
- DOI: 10.1128/JVI.00477-10
Phosphatidylinositol 3-kinase-, actin-, and microtubule-dependent transport of Semliki Forest Virus replication complexes from the plasma membrane to modified lysosomes
Abstract
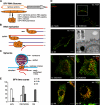
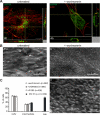
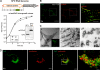
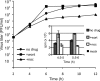
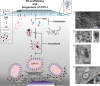
Like other positive-strand RNA viruses, alphaviruses replicate their genomes in association with modified intracellular membranes. Alphavirus replication sites consist of numerous bulb-shaped membrane invaginations (spherules), which contain the double-stranded replication intermediates. Time course studies with Semliki Forest virus (SFV)-infected cells were combined with live-cell imaging and electron microscopy to reveal that the replication complex spherules of SFV undergo an unprecedented large-scale movement between cellular compartments. The spherules first accumulated at the plasma membrane and were then internalized using an endocytic process that required a functional actin-myosin network, as shown by blebbistatin treatment. Wortmannin and other inhibitors indicated that the internalization of spherules also required the activity of phosphatidylinositol 3-kinase. The spherules therefore represent an unusual type of endocytic cargo. After endocytosis, spherule-containing vesicles were highly dynamic and had a neutral pH. These primary carriers fused with acidic endosomes and moved long distances on microtubules, in a manner prevented by nocodazole. The result of the large-scale migration was the formation of a very stable compartment, where the spherules were accumulated on the outer surfaces of unusually large and static acidic vacuoles localized in the pericentriolar region. Our work highlights both fundamental similarities and important differences in the processes that lead to the modified membrane compartments in cells infected by distinct groups of positive-sense RNA viruses.
Figures



References
-
- Acheson, N. H., and I. Tamm. 1967. Replication of Semliki Forest virus: an electron microscopic study. Virology 32:128-143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

