Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus
- PMID: 20484503
- PMCID: PMC2897609
- DOI: 10.1128/JVI.00479-10
Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus
Abstract
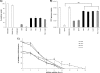
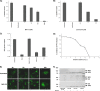
Porcine parvovirus (PPV) is a major cause of reproductive failure in swine. The mechanisms implicated in the first steps of infection that lead to the delivery of the PPV genome to the nucleus are poorly understood. In the present work, a panel of chemical inhibitors was used to dissect the cellular mechanisms involved in establishing a PPV infection. The results demonstrated that following binding to sialic acids on cell surface glycoproteins, the virus used both clathrin-mediated endocytosis and macropinocytosis pathways to gain access into cells. Virus obtained from infected cells was present either as isolated particles or as aggregates, and these two forms could be separated by low-speed centrifugation. Isolated and purified particles strongly preferred entry by clathrin-mediated endocytosis, whereas aggregates clearly favored macropinocytosis. Subsequent endosomal acidification and traffic to the late endosomes were also shown to be essential for infection. The microtubule network was found to be important during the first 10 h of infection, whereas an intact actin network was required for almost the whole viral cycle. Proteasome processing was found to be essential, and capsid proteins were ubiquitinated relatively early during infection. Taken together, these results provided new insights into the first steps of PPV infection, including the use of alternative entry pathways, unique among members of this viral family.
Figures

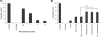

Similar articles
-
Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells.PLoS Pathog. 2009 Jul;5(7):e1000512. doi: 10.1371/journal.ppat.1000512. Epub 2009 Jul 10. PLoS Pathog. 2009. PMID: 19593382 Free PMC article.
-
Porcine Hemagglutinating Encephalomyelitis Virus Enters Neuro-2a Cells via Clathrin-Mediated Endocytosis in a Rab5-, Cholesterol-, and pH-Dependent Manner.J Virol. 2017 Nov 14;91(23):e01083-17. doi: 10.1128/JVI.01083-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956766 Free PMC article.
-
Rab5 and Rab11 Are Required for Clathrin-Dependent Endocytosis of Japanese Encephalitis Virus in BHK-21 Cells.J Virol. 2017 Sep 12;91(19):e01113-17. doi: 10.1128/JVI.01113-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724764 Free PMC article.
-
The parvovirus capsid odyssey: from the cell surface to the nucleus.Trends Microbiol. 2008 May;16(5):208-14. doi: 10.1016/j.tim.2008.01.012. Epub 2008 Apr 9. Trends Microbiol. 2008. PMID: 18406140 Review.
-
Structures and functions of parvovirus capsids and the process of cell infection.Curr Top Microbiol Immunol. 2010;343:149-76. doi: 10.1007/82_2010_33. Curr Top Microbiol Immunol. 2010. PMID: 20397069 Review.
Cited by
-
Porcine parvovirus replication is suppressed by activation of the PERK signaling pathway and endoplasmic reticulum stress-mediated apoptosis.Virology. 2020 Jan 2;539:1-10. doi: 10.1016/j.virol.2019.09.012. Epub 2019 Sep 26. Virology. 2020. PMID: 31605941 Free PMC article.
-
Sialoglycovirology of Lectins: Sialyl Glycan Binding of Enveloped and Non-enveloped Viruses.Methods Mol Biol. 2020;2132:483-545. doi: 10.1007/978-1-0716-0430-4_47. Methods Mol Biol. 2020. PMID: 32306355 Free PMC article.
-
Narrative Review of the Safety of Using Pigs for Xenotransplantation: Characteristics and Diagnostic Methods of Vertical Transmissible Viruses.Biomedicines. 2024 May 26;12(6):1181. doi: 10.3390/biomedicines12061181. Biomedicines. 2024. PMID: 38927388 Free PMC article. Review.
-
Proteomics analysis of PK-15 cells infected with porcine parvovirus and the effect of PCBP1 on PPV replication.Microbiol Spectr. 2024 Jun 4;12(6):e0391423. doi: 10.1128/spectrum.03914-23. Epub 2024 May 14. Microbiol Spectr. 2024. PMID: 38742903 Free PMC article.
-
Profiling of glycan receptors for minute virus of mice in permissive cell lines towards understanding the mechanism of cell recognition.PLoS One. 2014 Jan 27;9(1):e86909. doi: 10.1371/journal.pone.0086909. eCollection 2014. PLoS One. 2014. PMID: 24475195 Free PMC article.
References
-
- Bantel-Schaal, U., I. Braspenning-Wesch, and J. Kartenbeck. 2009. Adeno-associated virus type 5 exploits two different entry pathways in human embryo fibroblasts. J. Gen. Virol. 90:317-322. - PubMed
-
- Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. - PubMed
-
- Bergeron, J., J. Menezes, and P. Tijssen. 1993. Genomic organization and mapping of transcription and translation products of the NADL-2 strain of porcine parvovirus. Virology 197:86-98. - PubMed
-
- Bishop, N. E. 2003. Dynamics of endosomal sorting. Int. Rev. Cytol. 232:1-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

