Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization
- PMID: 20484519
- PMCID: PMC2897640
- DOI: 10.1128/JVI.01849-09
Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization
Abstract
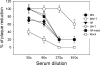
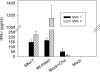
Influenza virus-like particles (VLPs) are a promising cell culture-based vaccine, and the skin is considered an attractive immunization site. In this study, we examined the immunogenicity and protective efficacy of influenza VLPs (H1N1 A/PR/8/34) after skin vaccination using vaccine dried on solid microneedle arrays. Coating of microneedles with influenza VLPs using an unstabilized formulation was found to decrease hemagglutinin (HA) activity, whereas inclusion of trehalose disaccharide preserved the HA activity of influenza VLP vaccines after microneedles were coated. Microneedle vaccination of mice in the skin with a single dose of stabilized influenza VLPs induced 100% protection against challenge infection with a high lethal dose. In contrast, unstabilized influenza VLPs, as well as intramuscularly injected vaccines, provided inferior immunity and only partial protection (</=40%). The stabilized microneedle vaccination group showed IgG2a levels that were 1 order of magnitude higher than those of other groups and had the lowest lung viral titers after challenge. Also, levels of recall immune responses, including hemagglutination inhibition titers, neutralizing antibodies, and antibody-secreting plasma cells, were significantly higher after skin vaccination with stabilized formulations. Therefore, our results indicate that HA stabilization, combined with vaccination via the skin using a vaccine formulated as a solid microneedle patch, confers protection superior to that with intramuscular injection and enables potential dose-sparing effects which are reflected by pronounced increases in rapid recall immune responses against influenza virus.
Figures





Similar articles
-
Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch.Clin Vaccine Immunol. 2013 Sep;20(9):1433-9. doi: 10.1128/CVI.00251-13. Epub 2013 Jul 17. Clin Vaccine Immunol. 2013. PMID: 23863506 Free PMC article.
-
Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles.J Control Release. 2010 Nov 1;147(3):326-32. doi: 10.1016/j.jconrel.2010.07.125. Epub 2010 Aug 6. J Control Release. 2010. PMID: 20692307 Free PMC article.
-
Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice.Clin Vaccine Immunol. 2010 Sep;17(9):1381-9. doi: 10.1128/CVI.00100-10. Epub 2010 Jul 14. Clin Vaccine Immunol. 2010. PMID: 20631330 Free PMC article.
-
Microneedle-based vaccines.Curr Top Microbiol Immunol. 2009;333:369-93. doi: 10.1007/978-3-540-92165-3_18. Curr Top Microbiol Immunol. 2009. PMID: 19768415 Free PMC article. Review.
-
Influenza vaccines based on virus-like particles.Virus Res. 2009 Aug;143(2):140-6. doi: 10.1016/j.virusres.2009.04.005. Epub 2009 Apr 15. Virus Res. 2009. PMID: 19374929 Free PMC article. Review.
Cited by
-
Virus-like particle vaccine protects against H3N2 canine influenza virus in dog.Vaccine. 2013 Jul 11;31(32):3268-73. doi: 10.1016/j.vaccine.2013.05.023. Epub 2013 May 22. Vaccine. 2013. PMID: 23707159 Free PMC article.
-
Progress in developing virus-like particle influenza vaccines.Expert Rev Vaccines. 2016 Oct;15(10):1281-93. doi: 10.1080/14760584.2016.1175942. Epub 2016 May 5. Expert Rev Vaccines. 2016. PMID: 27058302 Free PMC article. Review.
-
Progress in the development of virus-like particle vaccines against respiratory viruses.Expert Rev Vaccines. 2020 Jan;19(1):11-24. doi: 10.1080/14760584.2020.1711053. Epub 2020 Jan 18. Expert Rev Vaccines. 2020. PMID: 31903811 Free PMC article. Review.
-
Microneedle patches for vaccination in developing countries.J Control Release. 2016 Oct 28;240:135-141. doi: 10.1016/j.jconrel.2015.11.019. Epub 2015 Nov 18. J Control Release. 2016. PMID: 26603347 Free PMC article. Review.
-
Transdermal drug delivery systems for fighting common viral infectious diseases.Drug Deliv Transl Res. 2021 Aug;11(4):1498-1508. doi: 10.1007/s13346-021-01004-6. Epub 2021 May 22. Drug Deliv Transl Res. 2021. PMID: 34024014 Free PMC article. Review.
References
-
- Amorij, J. P., J. Meulenaar, W. L. Hinrichs, T. Stegmann, A. Huckriede, F. Coenen, and H. W. Frijlink. 2007. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine 25:6447-6457. - PubMed
-
- Anders, E. M., A. A. Scalzo, and D. O. White. 1985. Mitogenic activity of influenza virus and haemagglutinin. Vaccine 3:241-244. - PubMed
-
- Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. - PubMed
-
- Barker, J. N., R. S. Mitra, C. E. Griffiths, V. M. Dixit, and B. J. Nickoloff. 1991. Keratinocytes as initiators of inflammation. Lancet 337:211-214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

