Gain of function in FHM-1 Cav2.1 knock-in mice is related to the shape of the action potential
- PMID: 20484531
- PMCID: PMC2904224
- DOI: 10.1152/jn.00034.2010
Gain of function in FHM-1 Cav2.1 knock-in mice is related to the shape of the action potential
Abstract
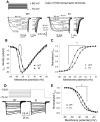
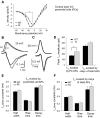
Familial hemiplegic migraine type-1 FHM-1 is caused by missense mutations in the CACNA1A gene that encodes the alpha(1A) pore-forming subunit of Ca(V)2.1 Ca(2+) channels. We used knock-in (KI) transgenic mice harboring the pathogenic FHM-1 mutation R192Q to study neurotransmission at the calyx of Held synapse and cortical layer 2/3 pyramidal cells (PCs). Using whole cell patch-clamp recordings in brain stem slices, we confirmed that KI Ca(V)2.1 Ca(2+) channels activated at more hyperpolarizing potentials. However, calyceal presynaptic calcium currents (I(pCa)) evoked by presynaptic action potentials (APs) were similar in amplitude, kinetic parameters, and neurotransmitter release. Ca(V)2.1 Ca(2+) channels in cortical layer 2/3 PCs from KI mice also showed a negative shift in their activation voltage. PCs had APs with longer durations and smaller amplitudes than the calyx of Held. AP-evoked Ca(2+) currents (I(Ca)) from PCs were larger in KI compared with wild-type (WT) mice. In contrast, when I(Ca)was evoked in PCs by calyx of Held AP waveforms, we observed no amplitude differences between WT and KI mice. In the same way, Ca(2+) currents evoked at the presynaptic terminals (I(pCa))of the calyx of Held by the AP waveforms of the PCs had larger amplitudes in R192Q KI mice that in WT. These results suggest that longer time courses of pyramidal APs were a key factor for the expression of a synaptic gain of function in the KI mice. In addition, our results indicate that consequences of FHM-1 mutations might vary according to the shape of APs in charge of triggering synaptic transmission (neurons in the calyx of Held vs. excitatory/inhibitory neurons in the cortex), adding to the complexity of the pathophysiology of migraine.
Figures



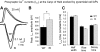
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

