The lateral paragigantocellular nucleus modulates parasympathetic cardiac neurons: a mechanism for rapid eye movement sleep-dependent changes in heart rate
- PMID: 20484535
- PMCID: PMC2934929
- DOI: 10.1152/jn.00228.2010
The lateral paragigantocellular nucleus modulates parasympathetic cardiac neurons: a mechanism for rapid eye movement sleep-dependent changes in heart rate
Abstract
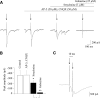
Rapid eye movement (REM) sleep is generally associated with a withdrawal of parasympathetic activity and heart rate increases; however, episodic vagally mediated heart rate decelerations also occur during REM sleep. This alternating pattern of autonomic activation provides a physiological basis for REM sleep-induced cardiac arrhythmias. Medullary neurons within the lateral paragigantocellular nucleus (LPGi) are thought to be active after REM sleep recovery and play a role in REM sleep control. In proximity to the LPGi are parasympathetic cardiac vagal neurons (CVNs) within the nucleus ambiguus (NA), which are critical for controlling heart rate. This study examined brain stem pathways that may mediate REM sleep-related reductions in parasympathetic cardiac activity. Electrical stimulation of the LPGi evoked inhibitory GABAergic postsynaptic currents in CVNs in an in vitro brain stem slice preparation in rats. Because brain stem cholinergic mechanisms are involved in REM sleep regulation, we also studied the role of nicotinic neurotransmission in modulation of GABAergic pathway from the LGPi to CVNs. Application of nicotine diminished the GABAergic responses evoked by electrical stimulation. This inhibitory effect of nicotine was prevented by the alpha7 nicotinic receptor antagonist alpha-bungarotoxin. Moreover, hypoxia/hypercapnia (H/H) diminished LPGi-evoked GABAergic current in CVNs, and this inhibitory effect was also prevented by alpha-bungarotoxin. In conclusion, stimulation of the LPGi evokes an inhibitory pathway to CVNs, which may constitute a mechanism for the reduced parasympathetic cardiac activity and increase in heart rate during REM sleep. Inhibition of this pathway by nicotinic receptor activation and H/H may play a role in REM sleep-related and apnea-associated bradyarrhythmias.
Figures
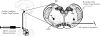





References
-
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla.J Comp Neurol 469: 391–412, 2004 - PubMed
-
- Benedito MA, Camarini R. Rapid eye movement sleep deprivation induces an increase in acetylcholinesterase activity in discrete rat brain regions.Braz J Med Biol Res 34: 103–109, 2001 - PubMed
-
- Berlad II, Shlitner A, Ben-Haim S, Lavie P. Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance.J Sleep Res 2: 88–90, 1993 - PubMed
-
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus.J Comp Neurol 262: 546–562, 1987 - PubMed
-
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study.Eur J Neurosci 16: 1959–1973, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

