Phosphatase of regenerating liver-1 promotes cell migration and invasion and regulates filamentous actin dynamics
- PMID: 20484558
- PMCID: PMC2913768
- DOI: 10.1124/jpet.110.167809
Phosphatase of regenerating liver-1 promotes cell migration and invasion and regulates filamentous actin dynamics
Abstract
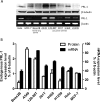
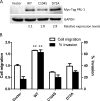
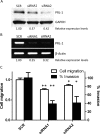
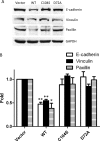
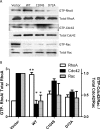
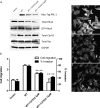
The phosphatases of regenerating liver (PRLs) are a unique family of plasma membrane-associated protein tyrosine phosphatases that have been hypothesized to be involved in metastatic cancer. How PRLs control cancer cell migration, invasion, and proliferation remains largely unknown. In the current study, we demonstrate a role for PRL-1 in the regulation of filamentous actin dynamics, which could promote cell metastatic processes. Human A549 non-small-cell lung cancer cells stably expressing wild-type PRL-1 exhibited a 60% increase in migration and a 3-fold increase in invasion. Cells expressing catalytic mutants of PRL-1 (C104S and D72A) lacked increased cell migration and invasion, indicating that these phenotypic changes required PRL-1 phosphatase activity. In contrast, PRL-1 small interfering RNA decreased in vitro lung cancer cell migration and invasion. The cadherin-catenin complex and dynamic filamentous actin are believed to control cellular invasiveness. Expression of wild-type PRL-1, but not phosphatase-inactive PRL-1 (C104S or D72A), decreased E-cadherin, vinculin, and paxillin expression. Ectopic expression of wild-type PRL-1 increased RhoA levels, which have an important role in actin filament assembly and stabilization of focal adhesion, and decreased activated Cdc42 and Rac. The Rho-associated protein kinase inhibitor, (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y-27632), decreased RhoA activity, actin filament levels, and cellular migration and invasion in PRL-1-expressing cells. These results suggest that PRL-1 could be a productive cancer therapeutic target and support further efforts to identify its substrates.
Figures
Similar articles
-
Phosphatase of regenerating liver: a novel target for cancer therapy.Expert Opin Ther Targets. 2014 May;18(5):555-69. doi: 10.1517/14728222.2014.892926. Epub 2014 Mar 1. Expert Opin Ther Targets. 2014. PMID: 24579927 Free PMC article. Review.
-
PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells.Cancer Res. 2007 Jan 15;67(2):643-50. doi: 10.1158/0008-5472.CAN-06-2436. Cancer Res. 2007. PMID: 17234774
-
PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility.Cancer Res. 2006 Mar 15;66(6):3153-61. doi: 10.1158/0008-5472.CAN-05-3116. Cancer Res. 2006. PMID: 16540666
-
Elevated phosphatase of regenerating liver 3 (PRL-3) promotes cytoskeleton reorganization, cell migration and invasion in endometrial stromal cells from endometrioma.Hum Reprod. 2016 Apr;31(4):723-33. doi: 10.1093/humrep/dew015. Epub 2016 Feb 13. Hum Reprod. 2016. PMID: 26874360
-
PRL phosphatases as potential molecular targets in cancer.Mol Cancer Ther. 2005 Nov;4(11):1653-61. doi: 10.1158/1535-7163.MCT-05-0248. Mol Cancer Ther. 2005. PMID: 16275986 Review.
Cited by
-
Migrastatics-Anti-metastatic and Anti-invasion Drugs: Promises and Challenges.Trends Cancer. 2017 Jun;3(6):391-406. doi: 10.1016/j.trecan.2017.04.008. Trends Cancer. 2017. PMID: 28670628 Free PMC article. Review.
-
PTP4A1 promotes oral squamous cell carcinoma (OSCC) metastasis through altered mitochondrial metabolic reprogramming.Cell Death Discov. 2023 Sep 29;9(1):360. doi: 10.1038/s41420-023-01657-x. Cell Death Discov. 2023. PMID: 37773151 Free PMC article.
-
The Leishmania donovani LDBPK_220120.1 Gene Encodes for an Atypical Dual Specificity Lipid-Like Phosphatase Expressed in Promastigotes and Amastigotes; Substrate Specificity, Intracellular Localizations, and Putative Role(s).Front Cell Infect Microbiol. 2021 Mar 25;11:591868. doi: 10.3389/fcimb.2021.591868. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842381 Free PMC article.
-
Phosphatase of Regenerating Liver-1 (PRL-1) Regulates Actin Dynamics During Immunological Synapse Assembly and T Cell Effector Function.Front Immunol. 2018 Nov 20;9:2655. doi: 10.3389/fimmu.2018.02655. eCollection 2018. Front Immunol. 2018. PMID: 30515156 Free PMC article.
-
Phosphatase of regenerating liver: a novel target for cancer therapy.Expert Opin Ther Targets. 2014 May;18(5):555-69. doi: 10.1517/14728222.2014.892926. Epub 2014 Mar 1. Expert Opin Ther Targets. 2014. PMID: 24579927 Free PMC article. Review.
References
-
- Achiwa H, Lazo JS. (2007) PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res 67:643–650 - PubMed
-
- Avizienyte E, Frame MC. (2005) Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol 17:542–547 - PubMed
-
- Bessette DC, Qiu D, Pallen CJ. (2008) PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev 27:231–252 - PubMed
-
- Burridge K, Wennerberg K. (2004) Rho and Rac take center stage. Cell 116:167–179 - PubMed
-
- Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, Crowell PL, Crowell DN. (1996) Prenylation of oncogenic human PTPCAAX protein tyrosine phosphatases. Cancer Lett 110:49–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

