Lens fiber cell differentiation and denucleation are disrupted through expression of the N-terminal nuclear receptor box of NCOA6 and result in p53-dependent and p53-independent apoptosis
- PMID: 20484573
- PMCID: PMC2903674
- DOI: 10.1091/mbc.e09-12-1031
Lens fiber cell differentiation and denucleation are disrupted through expression of the N-terminal nuclear receptor box of NCOA6 and result in p53-dependent and p53-independent apoptosis
Abstract
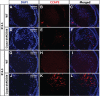
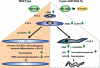
Nuclear receptor coactivator 6 (NCOA6) is a multifunctional protein implicated in embryonic development, cell survival, and homeostasis. An 81-amino acid fragment, dnNCOA6, containing the N-terminal nuclear receptor box (LXXLL motif) of NCOA6, acts as a dominant-negative (dn) inhibitor of NCOA6. Here, we expressed dnNCOA6 in postmitotic transgenic mouse lens fiber cells. The transgenic lenses showed reduced growth; a wide spectrum of lens fiber cell differentiation defects, including reduced expression of gamma-crystallins; and cataract formation. Those lens fiber cells entered an alternate proapoptotic pathway, and the denucleation (karyolysis) process was stalled. Activation of caspase-3 at embryonic day (E)13.5 was followed by double-strand breaks (DSBs) formation monitored via a biomarker, gamma-H2AX. Intense terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) signals were found at E16.5. Thus, a window of approximately 72 h between these events suggested prolonged though incomplete apoptosis in the lens fiber cell compartment that preserved nuclei in its cells. Genetic experiments showed that the apoptotic-like processes in the transgenic lens were both p53-dependent and p53-independent. Lens-specific deletion of Ncoa6 also resulted in disrupted lens fiber cell differentiation. Our data demonstrate a cell-autonomous role of Ncoa6 in lens fiber cell differentiation and suggest novel insights into the process of lens fiber cell denucleation and apoptosis.
Figures










References
-
- Alge C. S., Priglinger S. G., Neubauer A. S., Kampik A., Zillig M., Bloemendal H., Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest. Ophthalmol. Vis. Sci. 2002;43:3575–3582. - PubMed
-
- Andley U. P. Crystallins in the eye: function and pathology. Prog. Retin. Eye Res. 2007;26:78–98. - PubMed
-
- Anzick S. L., Kononen J., Walker R. L., Azorsa D. O., Tanner M. M., Guan X. Y., Sauter G., Kallioniemi O. P., Trent J. M., Meltzer P. S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

