Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light
- PMID: 20484624
- PMCID: PMC2883257
- DOI: 10.1523/JNEUROSCI.0613-10.2010
Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light
Abstract
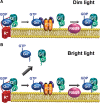
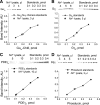
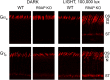
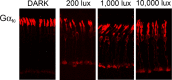
The remarkable ability of our vision to function under ever-changing conditions of ambient illumination is mediated by multiple molecular mechanisms regulating the light sensitivity of rods and cones. One such mechanism involves massive translocation of signaling proteins, including the G-protein transducin, into and out of the light-sensitive photoreceptor outer segment compartment. Transducin translocation extends the operating range of rods, but in cones transducin never translocates, which is puzzling because cones typically function in much brighter light than rods. Using genetically manipulated mice in which the rates of transducin activation and inactivation were altered, we demonstrate that, like in rods, transducin translocation in cones can be triggered when transducin activation exceeds a critical level, essentially saturating the photoresponse. However, this level is never achieved in wild-type cones: their superior ability to tightly control the rates of transducin activation and inactivation, responsible for avoiding saturation by light, also accounts for the prevention of transducin translocation at any light intensity.
Figures



Similar articles
-
Functional comparison of rod and cone Gα(t) on the regulation of light sensitivity.J Biol Chem. 2013 Feb 22;288(8):5257-67. doi: 10.1074/jbc.M112.430058. Epub 2013 Jan 3. J Biol Chem. 2013. PMID: 23288843 Free PMC article.
-
Rescue of cone function in cone-only Nphp5 knockout mouse model with Leber congenital amaurosis phenotype.Mol Vis. 2018 Dec 30;24:834-846. eCollection 2018. Mol Vis. 2018. PMID: 30713422 Free PMC article.
-
Low activation and fast inactivation of transducin in carp cones.J Biol Chem. 2012 Nov 30;287(49):41186-94. doi: 10.1074/jbc.M112.403717. Epub 2012 Oct 8. J Biol Chem. 2012. PMID: 23045532 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments.Vision Res. 2008 Feb;48(3):442-52. doi: 10.1016/j.visres.2007.08.020. Epub 2007 Oct 18. Vision Res. 2008. PMID: 17949773 Free PMC article. Review.
Cited by
-
The optoretinogram reveals the primary steps of phototransduction in the living human eye.Sci Adv. 2020 Sep 9;6(37):eabc1124. doi: 10.1126/sciadv.abc1124. Print 2020 Sep. Sci Adv. 2020. PMID: 32917686 Free PMC article.
-
Human retinal dark adaptation tracked in vivo with the electroretinogram: insights into processes underlying recovery of cone- and rod-mediated vision.J Physiol. 2022 Nov;600(21):4603-4621. doi: 10.1113/JP283105. Epub 2022 Jun 7. J Physiol. 2022. PMID: 35612091 Free PMC article. Review.
-
Autophagy supports color vision.Autophagy. 2015;11(10):1821-32. doi: 10.1080/15548627.2015.1084456. Autophagy. 2015. PMID: 26292183 Free PMC article.
-
Biochemistry and physiology of zebrafish photoreceptors.Pflugers Arch. 2021 Sep;473(9):1569-1585. doi: 10.1007/s00424-021-02528-z. Epub 2021 Feb 17. Pflugers Arch. 2021. PMID: 33598728 Free PMC article. Review.
-
Visual responses in mice lacking critical components of all known retinal phototransduction cascades.PLoS One. 2010 Nov 29;5(11):e15063. doi: 10.1371/journal.pone.0015063. PLoS One. 2010. PMID: 21124780 Free PMC article.
References
-
- Anant JS, Ong OC, Xie HY, Clarke S, O'Brien PJ, Fung BK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. - PubMed
-
- Arshavsky VY. Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci. 2002;25:124–126. - PubMed
-
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. - PubMed
-
- Artemyev NO. Light-dependent compartmentalization of transducin in rod photoreceptors. Mol Neurobiol. 2008;37:44–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY08571/EY/NEI NIH HHS/United States
- EY5722/EY/NEI NIH HHS/United States
- EY13729/EY/NEI NIH HHS/United States
- P30 EY005722/EY/NEI NIH HHS/United States
- R01 EY010336/EY/NEI NIH HHS/United States
- EY11123/EY/NEI NIH HHS/United States
- U10 EY013729/EY/NEI NIH HHS/United States
- EY072224/EY/NEI NIH HHS/United States
- P30 EY008571/EY/NEI NIH HHS/United States
- EY10336/EY/NEI NIH HHS/United States
- R01 EY011123/EY/NEI NIH HHS/United States
- P30 EY021721/EY/NEI NIH HHS/United States
- R01 EY012224/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
