MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma
- PMID: 20484625
- PMCID: PMC2883258
- DOI: 10.1523/JNEUROSCI.6239-09.2010
MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma
Abstract
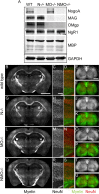
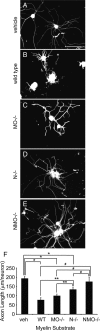
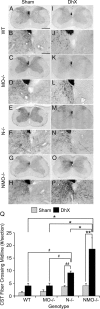
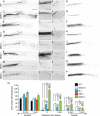
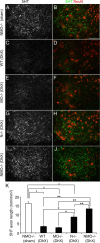
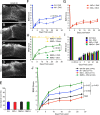
Functional recovery after adult CNS damage is limited in part by myelin inhibitors of axonal regrowth. Three molecules, Nogo-A, MAG, and OMgp, are produced by oligodendrocytes and share neuronal receptor mechanisms through NgR1 and PirB. While each has an axon-inhibitory role in vitro, their in vivo interactions and relative potencies have not been defined. Here, we compared mice singly, doubly, or triply mutant for these three myelin inhibitor proteins. The myelin extracted from Nogo-A mutant mice is less inhibitory for axons than is that from wild-type mice, but myelin lacking MAG and OMgp is indistinguishable from control. However, myelin lacking all three inhibitors is less inhibitory than Nogo-A-deficient myelin, uncovering a redundant and synergistic role for all three proteins in axonal growth inhibition. Spinal cord injury studies revealed an identical in vivo hierarchy of these three myelin proteins. Loss of Nogo-A allows corticospinal and raphespinal axon growth above and below the injury, as well as greater behavioral recovery than in wild-type or heterozygous mutant mice. In contrast, deletion of MAG and OMgp stimulates neither axonal growth nor enhanced locomotion. The triple-mutant mice exhibit greater axonal growth and improved locomotion, consistent with a principal role for Nogo-A and synergistic actions for MAG and OMgp, presumably through shared receptors. These data support the hypothesis that targeting all three myelin ligands, as with NgR1 decoy receptor, provides the optimal chance for overcoming myelin inhibition and improving neurological function.
Figures


References
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Bartsch U. Myelination and axonal regeneration in the central nervous system of mice deficient in the myelin-associated glycoprotein. J Neurocytol. 1996;25:303–313. - PubMed
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for Locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
