The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1
- PMID: 20484627
- PMCID: PMC2887690
- DOI: 10.1523/JNEUROSCI.0761-10.2010
The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1
Abstract
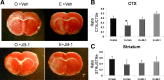
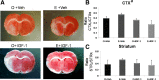
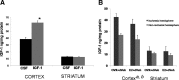
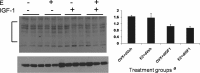
Hormone therapy to postmenopausal females increases the risk and severity of ischemic stroke. Our previous work using an animal model of menopause (reproductive senescence) shows that middle cerebral artery occlusion (MCAo) causes a larger cortical-striatal infarct in this older acyclic group compared with younger females. Moreover, although estrogen treatment is neuroprotective in younger females, estrogen paradoxically increases infarct volume in acyclic females. We hypothesized that the neurotoxic effects of estrogen in older females occurs because of decreased availability of IGF-1, a neuroprotectant that decreases with advancing age and is downregulated by estrogen treatment. Our data show that plasma IGF-1 levels are significantly reduced in reproductive senescent females and further reduced by estrogen at all ages. The neuroprotective effect of estrogen on MCAo-induced cortical infarct volume in mature adult female is reversed by intracerebroventricular injections of IGF-1 receptor antagonist JB-1. Similarly, estrogens neurotoxic effects on cortical infarct volume in senescent females is attenuated by concurrent IGF-1 treatment, and reversed when IGF-1 is infused 4 h after the onset of ischemia (delayed IGF-1 treatment). Delayed IGF-1/estrogen treatment also suppressed ischemia-induced ERK1 phosphorylation, reduced protein oxidation, and stimulated an early increase in prostaglandin E(2) at the infarct site. IGF-1 treatment was only protective in senescent females that received estrogen, indicating that the neuroprotective actions of this peptide require interaction with the steroid hormone receptor. These data support the hypothesis that stroke severity in older females is associated with decreased IGF-1 and further indicate that short-term postischemic IGF-1 therapy may be beneficial for stroke.
Figures



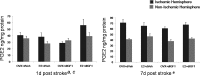

References
-
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. - PubMed
-
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. - PubMed
-
- Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med. 2001;46:827–830. - PubMed
-
- Böttner M, Wuttke W. Chronic treatment with physiological doses of estradiol affects the GH-IGF-1 axis and fat metabolism in young and middle-aged ovariectomized rats. Biogerontology. 2006;7:91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
