Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer's disease
- PMID: 20484629
- PMCID: PMC6632652
- DOI: 10.1523/JNEUROSCI.0519-10.2010
Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer's disease
Abstract
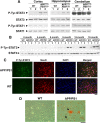
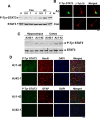
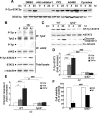
One of the pathological hallmarks of Alzheimer's disease (AD) is deposition of extracellular amyloid-beta (Abeta) peptide, which is generated from the cleavage of amyloid precursor protein (APP). Accumulation of Abeta is thought to associate with the progressive neuronal death observed in AD. However, the precise signaling mechanisms underlying the action of Abeta in AD pathophysiology are not completely understood. Here, we report the involvement of the transcription factor signal transducer and activator of transcription 3 (STAT3) in mediating Abeta-induced neuronal death. We find that tyrosine phosphorylation of STAT3 is elevated in the cortex and hippocampus of APP/PS1 transgenic mice. Treatment of cultured rat neurons with Abeta or intrahippocampal injection of mice with Abeta both induces tyrosine phosphorylation of STAT3 in neurons. Importantly, reduction of either the expression or activation of STAT3 markedly attenuates Abeta-induced neuronal apoptosis, suggesting that STAT3 activation contributes to neuronal death after Abeta exposure. We further identify Tyk2 as the tyrosine kinase that acts upstream of STAT3, as Abeta-induced activation of STAT3 and caspase-3-dependent neuronal death can be inhibited in tyk2(-/-) neurons. Finally, increased tyrosine phosphorylation of STAT3 is also observed in postmortem brains of AD patients. Our observations collectively reveal a novel role of STAT3 in Abeta-induced neuronal death and suggest the potential involvement of Tyk2/STAT3 signaling in AD pathophysiology.
Figures



References
-
- Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. - PubMed
-
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. - PubMed
-
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
