Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling
- PMID: 20484632
- PMCID: PMC2879010
- DOI: 10.1523/JNEUROSCI.0527-10.2010
Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling
Abstract
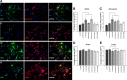
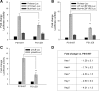
The vast majority of pedigrees with familial Alzheimer's disease (FAD) are caused by inheritance of mutations in the PSEN1 1 gene. While genetic ablation studies have revealed a role for presenilin 1 (PS1) in embryonic neurogenesis, little information has emerged regarding the potential effects of FAD-linked PS1 variants on proliferation, self-renewal and differentiation, key events that control cell fate commitment of adult brain neural progenitors (NPCs). We used adult brain subventricular zone (SVZ)-derived NPC cultures transduced with recombinant lentivirus as a means to investigate the effects of various PS1 mutants on self-renewal and differentiation properties. We now show that viral expression of several PS1 mutants in NPCs leads to impaired self-renewal and altered differentiation toward neuronal lineage, in vitro. In line with these observations, diminished constitutive proliferation and steady-state SVZ progenitor pool size was observed in vivo in transgenic mice expressing the PS1DeltaE9 variant. Moreover, NPC cultures established from the SVZ of adult mice expressing PS1DeltaE9 exhibit reduced self-renewal capacity and premature exit toward neuronal fates. To these findings, we show that both the levels of endogenous Notch/CBF-1-transcriptional activity and transcripts encoding Notch target genes are diminished in SVZ NPCs expressing PS1DeltaE9. The deficits in self-renewal and multipotency are restored by expression of Notch1-ICD or a downstream target of the Notch pathway, Hes1. Hence, we argue that a partial reduction in PS-dependent gamma-secretase processing of the Notch, at least in part, accounts for the impairments observed in SVZ NPCs expressing the FAD-linked PS1DeltaE9 variant.
Figures





Similar articles
-
Mutant presenilin 1 expression in excitatory neurons impairs enrichment-mediated phenotypes of adult hippocampal progenitor cells.Proc Natl Acad Sci U S A. 2013 May 28;110(22):9148-53. doi: 10.1073/pnas.1302106110. Epub 2013 May 14. Proc Natl Acad Sci U S A. 2013. PMID: 23674689 Free PMC article.
-
Deficits in Enrichment-Dependent Neurogenesis and Enhanced Anxiety Behaviors Mediated by Expression of Alzheimer's Disease-Linked Ps1 Variants Are Rescued by Microglial Depletion.J Neurosci. 2019 Aug 21;39(34):6766-6780. doi: 10.1523/JNEUROSCI.0884-19.2019. Epub 2019 Jun 19. J Neurosci. 2019. PMID: 31217332 Free PMC article.
-
Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal.Nature. 2010 Sep 16;467(7313):323-7. doi: 10.1038/nature09347. Nature. 2010. PMID: 20844536 Free PMC article.
-
Notch: from neural development to neurological disorders.J Neurochem. 2008 Dec;107(6):1471-81. doi: 10.1111/j.1471-4159.2008.05715.x. J Neurochem. 2008. PMID: 19094054 Free PMC article. Review.
-
Alteration of Notch signaling in skeletal development and disease.Ann N Y Acad Sci. 2010 Mar;1192:257-68. doi: 10.1111/j.1749-6632.2009.05307.x. Ann N Y Acad Sci. 2010. PMID: 20392245 Free PMC article. Review.
Cited by
-
γ-Secretase modulators reduce endogenous amyloid β42 levels in human neural progenitor cells without altering neuronal differentiation.FASEB J. 2015 Aug;29(8):3335-41. doi: 10.1096/fj.15-271015. Epub 2015 Apr 22. FASEB J. 2015. PMID: 25903103 Free PMC article.
-
Ginkgo biloba extract improves cognitive function and increases neurogenesis by reducing Aβ pathology in 5×FAD mice.Am J Transl Res. 2021 Mar 15;13(3):1471-1482. eCollection 2021. Am J Transl Res. 2021. PMID: 33841671 Free PMC article.
-
Adult human neurogenesis: from microscopy to magnetic resonance imaging.Front Neurosci. 2011 Apr 4;5:47. doi: 10.3389/fnins.2011.00047. eCollection 2011. Front Neurosci. 2011. PMID: 21519376 Free PMC article.
-
Mitochondria and neuroplasticity.ASN Neuro. 2010 Oct 4;2(5):e00045. doi: 10.1042/AN20100019. ASN Neuro. 2010. PMID: 20957078 Free PMC article. Review.
-
Not(ch) just development: Notch signalling in the adult brain.Nat Rev Neurosci. 2011 May;12(5):269-83. doi: 10.1038/nrn3024. Nat Rev Neurosci. 2011. PMID: 21505516 Free PMC article. Review.
References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
-
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horré K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. - PubMed
-
- Berechid BE, Kitzmann M, Foltz DR, Roach AH, Seiffert D, Thompson LA, Olson RE, Bernstein A, Donoviel DB, Nye JS. Identification and characterization of presenilin-independent Notch signaling. J Biol Chem. 2002;277:8154–8165. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
