Rac1 regulates neuronal polarization through the WAVE complex
- PMID: 20484635
- PMCID: PMC6632643
- DOI: 10.1523/JNEUROSCI.5395-09.2010
Rac1 regulates neuronal polarization through the WAVE complex
Abstract
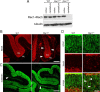
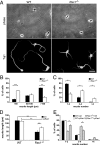
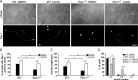
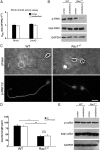
Neuronal migration and axon growth, key events during neuronal development, require distinct changes in the cytoskeleton. Although many molecular regulators of polarity have been identified and characterized, relatively little is known about their physiological role in this process. To study the physiological function of Rac1 in neuronal development, we have generated a conditional knock-out mouse, in which Rac1 is ablated in the whole brain. Rac1-deficient cerebellar granule neurons, which do not express other Rac isoforms, showed impaired neuronal migration and axon formation both in vivo and in vitro. In addition, Rac1 ablation disrupts lamellipodia formation in growth cones. The analysis of Rac1 effectors revealed the absence of the Wiskott-Aldrich syndrome protein (WASP) family verprolin-homologous protein (WAVE) complex from the plasma membrane of knock-out growth cones. Loss of WAVE function inhibited axon growth, whereas overexpression of a membrane-tethered WAVE mutant partially rescued axon growth in Rac1-knock-out neurons. In addition, pharmacological inhibition of the WAVE complex effector Arp2/3 also reduced axon growth. We propose that Rac1 recruits the WAVE complex to the plasma membrane to enable actin remodeling necessary for axon growth.
Figures




References
-
- Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277:37771–37776. - PubMed
-
- Bogdan S, Grewe O, Strunk M, Mertens A, Klämbt C. Sra-1 interacts with Kette and Wasp and is required for neuronal and bristle development in Drosophila. Development. 2004;131:3981–3989. - PubMed
-
- Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. - PubMed
-
- Bolis A, Corbetta S, Cioce A, de Curtis I. Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: implications for a role of Rac3 in Purkinje cell differentiation. Eur J Neurosci. 2003;18:2417–2424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
