Assembly of large-area, highly ordered, crack-free inverse opal films
- PMID: 20484675
- PMCID: PMC2890797
- DOI: 10.1073/pnas.1000954107
Assembly of large-area, highly ordered, crack-free inverse opal films
Abstract
Whereas considerable interest exists in self-assembly of well-ordered, porous "inverse opal" structures for optical, electronic, and (bio)chemical applications, uncontrolled defect formation has limited the scale-up and practicality of such approaches. Here we demonstrate a new method for assembling highly ordered, crack-free inverse opal films over a centimeter scale. Multilayered composite colloidal crystal films have been generated via evaporative deposition of polymeric colloidal spheres suspended within a hydrolyzed silicate sol-gel precursor solution. The coassembly of a sacrificial colloidal template with a matrix material avoids the need for liquid infiltration into the preassembled colloidal crystal and minimizes the associated cracking and inhomogeneities of the resulting inverse opal films. We discuss the underlying mechanisms that may account for the formation of large-area defect-free films, their unique preferential growth along the 110 direction and unusual fracture behavior. We demonstrate that this coassembly approach allows the fabrication of hierarchical structures not achievable by conventional methods, such as multilayered films and deposition onto patterned or curved surfaces. These robust SiO(2) inverse opals can be transformed into various materials that retain the morphology and order of the original films, as exemplified by the reactive conversion into Si or TiO(2) replicas. We show that colloidal coassembly is available for a range of organometallic sol-gel and polymer matrix precursors, and represents a simple, low-cost, scalable method for generating high-quality, chemically tailorable inverse opal films for a variety of applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
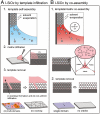




 at 200 °C for 2 h and with water vapor at 400 °C for 5 h.
at 200 °C for 2 h and with water vapor at 400 °C for 5 h.References
-
- Holland BT, Blanford CF, Stein A. Synthesis of macroporous minerals with highly ordered three-dimensional arrays of spheroidal voids. Science. 1998;281:538–540. - PubMed
-
- Lytle JC, Stein A. Recent progress in synthesis and applications of inverse opals and related macroporous materials prepared by colloidal crystal templating. In: Cao G, Brinker CJ, editors. Ann Rev Nano Res. Vol. 1. Teaneck, NJ: World Scientific; 2006. pp. 1–79.
-
- Velev OD, Lenhoff AM. Colloidal crystals as templates for porous materials. Curr Opin Colloid Interface Sci. 2000;5:56–63.
-
- Xia Y, Gates B, Yin Y, Lu Y. Monodispersed colloidal spheres: old materials with new applications. Adv Mater. 2000;12:693–713.
-
- Zhao XS, et al. Templating methods for preparation of porous structures. J Mater Chem. 2006;16:637–648.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

