Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles
- PMID: 20484700
- PMCID: PMC2928615
- DOI: 10.1152/ajpregu.00111.2010
Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles
Abstract
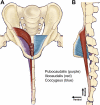
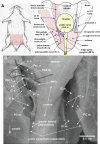
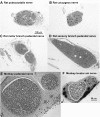
The urethral rhabdosphincter and pelvic floor muscles are important in maintenance of urinary continence and in preventing descent of pelvic organs [i.e., pelvic organ prolapse (POP)]. Despite its clinical importance and complexity, a comprehensive review of neural control of the rhabdosphincter and pelvic floor muscles is lacking. The present review places historical and recent basic science findings on neural control into the context of functional anatomy of the pelvic muscles and their coordination with visceral function and correlates basic science findings with clinical findings when possible. This review briefly describes the striated muscles of the pelvis and then provides details on the peripheral innervation and, in particular, the contributions of the pudendal and levator ani nerves to the function of the various pelvic muscles. The locations and unique phenotypic characteristics of rhabdosphincter motor neurons located in Onuf's nucleus, and levator ani motor neurons located diffusely in the sacral ventral horn, are provided along with the locations and phenotypes of primary afferent neurons that convey sensory information from these muscles. Spinal and supraspinal pathways mediating excitatory and inhibitory inputs to the motor neurons are described; the relative contributions of the nerves to urethral function and their involvement in POP and incontinence are discussed. Finally, a detailed summary of the neurochemical anatomy of Onuf's nucleus and the pharmacological control of the rhabdosphincter are provided.
Figures
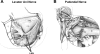






References
-
- Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol 97: 770–779, 1990 - PubMed
-
- Arvidsson U, Risling M, Frisen J, Piehl F, Fried K, Hokfelt T, Cullheim S. trkC-Like immunoreactivity in the primate descending serotoninergic system. Eur J Neurosci 6: 230–236, 1994 - PubMed
-
- Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann NY Acad Sci 1101: 266–296, 2007 - PubMed
-
- Barber MD, Bremer RE, Thor KB, Dolber PC, Kuehl TJ, Coates KW. Innervation of the female levator ani muscles. Am J Obstet Gynecol 187: 64–71, 2002 - PubMed
-
- Beattie MS, Li Q, Leedy MG, Bresnahan JC. Motoneurons innervating the external anal and urethral sphincters of the female cat have different patterns of dendritic arborization. Neurosci Lett 111: 69–74, 1990 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

