The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice
- PMID: 20484814
- PMCID: PMC2877958
- DOI: 10.1172/JCI42164
The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice
Abstract
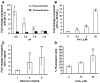
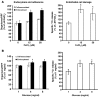
Mucormycosis is a fungal infection of the sinuses, brain, or lungs that causes a mortality rate of at least 50% despite first-line therapy. Because angioinvasion is a hallmark of mucormycosis infections, we sought to define the endothelial cell receptor(s) for fungi of the order Mucorales (the fungi that cause mucormycosis). Furthermore, since patients with elevated available serum iron, including those with diabetic ketoacidosis (DKA), are uniquely susceptible to mucormycosis, we sought to define the role of iron and glucose in regulating the expression of such a receptor. Here, we have identified glucose-regulated protein 78 (GRP78) as what we believe to be a novel host receptor that mediates invasion and damage of human endothelial cells by Rhizopus oryzae, the most common etiologic species of Mucorales, but not Candida albicans or Aspergillus fumigatus. Elevated concentrations of glucose and iron, consistent with those seen during DKA, enhanced GRP78 expression and the resulting R. oryzae invasion and damage of endothelial cells in a receptor-dependent manner. Mice with DKA, which have enhanced susceptibility to mucormycosis, exhibited increased expression of GRP78 in sinus, lungs, and brain compared with normal mice. Finally, GRP78-specific immune serum protected mice with DKA from mucormycosis. These results suggest a unique susceptibility of patients with DKA to mucormycosis and provide a foundation for the development of new therapeutic interventions for these deadly infections.
Figures

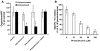
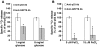

Similar articles
-
Host-iron assimilation: pathogenesis and novel therapies of mucormycosis.Mycoses. 2014 Dec;57 Suppl 3(0 3):13-7. doi: 10.1111/myc.12232. Epub 2014 Sep 1. Mycoses. 2014. PMID: 25178879 Free PMC article. Review.
-
GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis.mBio. 2020 Jun 2;11(3):e01087-20. doi: 10.1128/mBio.01087-20. mBio. 2020. PMID: 32487760 Free PMC article.
-
Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis.J Clin Invest. 2016 Jun 1;126(6):2280-94. doi: 10.1172/JCI82744. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159390 Free PMC article.
-
CotH3 mediates fungal invasion of host cells during mucormycosis.J Clin Invest. 2014 Jan;124(1):237-50. doi: 10.1172/JCI71349. Epub 2013 Dec 20. J Clin Invest. 2014. PMID: 24355926 Free PMC article.
-
Pathogenesis of mucormycosis.Clin Infect Dis. 2012 Feb;54 Suppl 1(Suppl 1):S16-22. doi: 10.1093/cid/cir865. Clin Infect Dis. 2012. PMID: 22247441 Free PMC article. Review.
Cited by
-
Mucormycosis during COVID-19 era: A retrospective assessment.Infect Med (Beijing). 2024 Apr 15;3(2):100112. doi: 10.1016/j.imj.2024.100112. eCollection 2024 Jun. Infect Med (Beijing). 2024. PMID: 38948388 Free PMC article. Review.
-
ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries.Mycoses. 2021 Sep;64(9):1028-1037. doi: 10.1111/myc.13335. Epub 2021 Jul 26. Mycoses. 2021. PMID: 34133816 Free PMC article.
-
Case Report: Rhino-orbital Mucormycosis Related to COVID-19: A Case Series Exploring Risk Factors.Am J Trop Med Hyg. 2021 Dec 13;106(2):566-570. doi: 10.4269/ajtmh.21-0777. Am J Trop Med Hyg. 2021. PMID: 34902834 Free PMC article.
-
Host-iron assimilation: pathogenesis and novel therapies of mucormycosis.Mycoses. 2014 Dec;57 Suppl 3(0 3):13-7. doi: 10.1111/myc.12232. Epub 2014 Sep 1. Mycoses. 2014. PMID: 25178879 Free PMC article. Review.
-
Molecular mechanisms of mucormycosis-The bitter and the sweet.PLoS Pathog. 2017 Aug 3;13(8):e1006408. doi: 10.1371/journal.ppat.1006408. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28771587 Free PMC article. Review. No abstract available.
References
-
- Ibrahim AS, Edwards JE, Filler SG. Zygomycoses. In: Dismukes WE, Pappas PG, Sobel JD, eds.Clinical Mycology . New York, NY: Oxford University Press; 2003:241–251.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

