A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium
- PMID: 20484817
- PMCID: PMC2877937
- DOI: 10.1172/JCI40253
A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium
Abstract
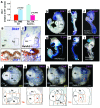
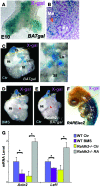
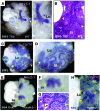
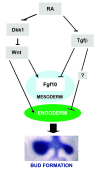
The developmental abnormalities associated with disruption of signaling by retinoic acid (RA), the biologically active form of vitamin A, have been known for decades from studies in animal models and humans. These include defects in the respiratory system, such as lung hypoplasia and agenesis. However, the molecular events controlled by RA that lead to formation of the lung primordium from the primitive foregut remain unclear. Here, we present evidence that endogenous RA acts as a major regulatory signal integrating Wnt and Tgfbeta pathways in the control of Fgf10 expression during induction of the mouse primordial lung. We demonstrated that activation of Wnt signaling required for lung formation was dependent on local repression of its antagonist, Dickkopf homolog 1 (Dkk1), by endogenous RA. Moreover, we showed that simultaneously activating Wnt and repressing Tgfbeta allowed induction of both lung buds in RA-deficient foreguts. The data in this study suggest that disruption of Wnt/Tgfbeta/Fgf10 interactions represents the molecular basis for the classically reported failure to form lung buds in vitamin A deficiency.
Figures



References
-
- Gavrilova R, et al. Vitamin A deficiency in an infant with PAGOD syndrome. Am J Med Genet A. 2009;149A(10):2241–2247. - PubMed
-
- Kastner P, et al. Genetic evidence that the retinoid signaling is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124(2):313–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

