NHE4 is critical for the renal handling of ammonia in rodents
- PMID: 20484819
- PMCID: PMC2877923
- DOI: 10.1172/JCI36581
NHE4 is critical for the renal handling of ammonia in rodents
Abstract
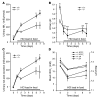
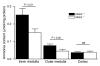
Ammonia absorption by the medullary thick ascending limb of Henle's loop (MTALH) is thought to be a critical step in renal ammonia handling and excretion in urine, in which it is the main acid component. Basolateral Na+/H+ exchangers have been proposed to play a role in ammonia efflux out of MTALH cells, which express 2 exchanger isoforms: Na+/H+ exchanger 1 (NHE1) and NHE4. Here, we investigated the role of NHE4 in urinary acid excretion and found that NHE4-/- mice exhibited compensated hyperchloremic metabolic acidosis, together with inappropriate urinary net acid excretion. When challenged with a 7-day HCl load, NHE4-/- mice were unable to increase their urinary ammonium and net acid excretion and displayed reduced ammonium medulla content compared with wild-type littermates. Both pharmacologic inhibition and genetic disruption of NHE4 caused a marked decrease in ammonia absorption by the MTALH. Finally, dietary induction of metabolic acidosis increased NHE4 mRNA expression in mouse MTALH cells and enhanced renal NHE4 activity in rats, as measured by in vitro microperfusion of MTALH. We therefore conclude that ammonia absorption by the MTALH requires the presence of NHE4 and that lack of NHE4 reduces the ability of MTALH epithelial cells to create the cortico-papillary gradient of NH3/NH4+ needed to excrete an acid load, contributing to systemic metabolic acidosis.
Figures
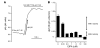
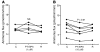


Similar articles
-
Lack of a role of NHE4 in renal ammonia metabolism.Am J Physiol Renal Physiol. 2025 Jun 1;328(6):F752-F765. doi: 10.1152/ajprenal.00044.2025. Epub 2025 Apr 16. Am J Physiol Renal Physiol. 2025. PMID: 40235211 Free PMC article.
-
More actors in ammonia absorption by the thick ascending limb.Am J Physiol Renal Physiol. 2012 Feb 1;302(3):F293-7. doi: 10.1152/ajprenal.00307.2011. Epub 2011 Nov 16. Am J Physiol Renal Physiol. 2012. PMID: 22088435 Review.
-
Role of NH3 and NH4+ transporters in renal acid-base transport.Am J Physiol Renal Physiol. 2011 Jan;300(1):F11-23. doi: 10.1152/ajprenal.00554.2010. Epub 2010 Nov 3. Am J Physiol Renal Physiol. 2011. PMID: 21048022 Free PMC article. Review.
-
Localization and functional characterization of Na+/H+ exchanger isoform NHE4 in rat thick ascending limbs.Am J Physiol Renal Physiol. 2001 Oct;281(4):F707-17. doi: 10.1152/ajprenal.2001.281.4.F707. Am J Physiol Renal Physiol. 2001. PMID: 11553518
-
Ammonium transport in the kidney.J Nephrol. 2010 Nov-Dec;23 Suppl 16:S28-34. J Nephrol. 2010. PMID: 21170885 Review.
Cited by
-
Sulfatides are required for renal adaptation to chronic metabolic acidosis.Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):9998-10003. doi: 10.1073/pnas.1217775110. Epub 2013 May 28. Proc Natl Acad Sci U S A. 2013. PMID: 23716689 Free PMC article.
-
The Na+/H+ Exchanger 3 in the Intestines and the Proximal Tubule of the Kidney: Localization, Physiological Function, and Key Roles in Angiotensin II-Induced Hypertension.Front Physiol. 2022 Apr 19;13:861659. doi: 10.3389/fphys.2022.861659. eCollection 2022. Front Physiol. 2022. PMID: 35514347 Free PMC article. Review.
-
Emerging Features of Ammonia Metabolism and Transport in Acid-Base Balance.Semin Nephrol. 2019 Jul;39(4):394-405. doi: 10.1016/j.semnephrol.2019.04.008. Semin Nephrol. 2019. PMID: 31300094 Free PMC article. Review.
-
Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion.Clin J Am Soc Nephrol. 2015 Aug 7;10(8):1444-58. doi: 10.2215/CJN.10311013. Epub 2014 Jul 30. Clin J Am Soc Nephrol. 2015. PMID: 25078422 Free PMC article. Review.
-
Cation-coupled bicarbonate transporters.Compr Physiol. 2014 Oct;4(4):1605-37. doi: 10.1002/cphy.c130005. Compr Physiol. 2014. PMID: 25428855 Free PMC article. Review.
References
-
- Good DW. Adaptation of HCO3– and NH4+ transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Am J Physiol. 1990;258(5 pt 2):F1345–F1353. - PubMed
-
- Packer RK, Desai SS, Hornbuckle K, Knepper MA. Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J Am Soc Nephrol. 1991;2(1):77–83. - PubMed
-
- Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984;247(1 pt 2):F35–F44. - PubMed
-
- Kinne R, Kinne Saffran E, Schutz H, Scholermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl(-)-cotransporter. J Membr Biol. 1986;94(3):279–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

