PET probes for distinct metabolic pathways have different cell specificities during immune responses in mice
- PMID: 20484820
- PMCID: PMC2877561
- DOI: 10.1172/JCI41250
PET probes for distinct metabolic pathways have different cell specificities during immune responses in mice
Erratum in
- J Clin Invest. 2010 Jul 1;120(7):2641
Abstract
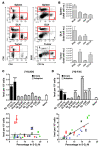
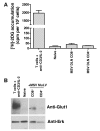
Clinical tools that measure changes in immune cell metabolism would improve the diagnosis and treatment of immune dysfunction. PET, utilizing probes for specific metabolic processes, detects regions of immune activation in vivo. In this study we investigated the immune cell specificity of PET probes for two different metabolic pathways: [18F]-2-fluorodeoxyglucose ([18F]-FDG) for glycolysis and [18F]-2-fluoro-D-(arabinofuranosyl)cytosine ([18F]-FAC) for deoxycytidine salvage. We isolated innate and adaptive immune cells from tissues of mice challenged with a retrovirus-induced sarcoma and measured their ability to accumulate FDG and FAC. We determined that the two probes had distinct patterns of accumulation: FDG accumulated to the highest levels in innate immune cells, while FAC accumulated predominantly in CD8+ T cells in a manner that correlated with cellular proliferation. This study demonstrates that innate and adaptive cell types differ in glycolytic and deoxycytidine salvage demands during an immune response and that these differential metabolic requirements can be detected with specific PET probes. Our findings have implications for the interpretation of clinical PET scans that use [18F]-FDG or [18F]-FAC to assess immune function in vivo and suggest potential applications of metabolic PET to monitor the effects of targeted immune modulation.
Figures



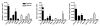

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

