Designing customized cell signalling circuits
- PMID: 20485291
- PMCID: PMC2975372
- DOI: 10.1038/nrm2904
Designing customized cell signalling circuits
Abstract
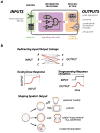
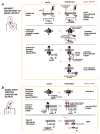
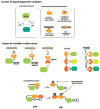
Living cells have evolved a broad array of complex signalling responses, which enables them to survive diverse environmental challenges and execute specific physiological functions. Our increasingly sophisticated understanding of the molecular mechanisms of cell signalling networks in eukaryotes has revealed a remarkably modular organization and synthetic biologists are exploring how this can be exploited to engineer cells with novel signalling behaviours. This approach is beginning to reveal the logic of how cells might evolve innovative new functions and moves us towards the exciting possibility of engineering custom cells with precise sensing-response functions that could be useful in medicine and biotechnology.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

