Autoimmunity at the ocular surface: pathogenesis and regulation
- PMID: 20485329
- PMCID: PMC3577924
- DOI: 10.1038/mi.2010.26
Autoimmunity at the ocular surface: pathogenesis and regulation
Abstract
A healthy ocular surface environment is essential to preserve visual function, and as such the eye has evolved a complex network of mechanisms to maintain homeostasis. Fundamental to the health of the ocular surface is the immune system, designed to respond rapidly to environmental and microbial insults, whereas maintaining tolerance to self-antigens and commensal microbes. To this end, activation of the innate and adaptive immune response is tightly regulated to limit bystander tissue damage. However, aberrant activation of the immune system can result in autoimmunity to self-antigens localized to the ocular surface and associated tissues. Environmental, microbial and endogenous stress, antigen localization, and genetic factors provide the triggers underlying the immunological events that shape the outcome of the diverse spectrum of autoimmune-based ocular surface disorders.
Figures
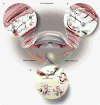
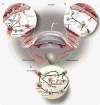
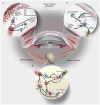
References
-
- Stern ME, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. - PubMed
-
- Beuerman R, Mircheff AK, Pflugfelder SC, Stern ME. The lacrimal functional unit. In: Pflugfelder SC, Beuerman RW, Stern ME, editors. Dry Eye and Ocular Surface Disorders. Marchel Dekker; New York: 2004. pp. 11–39.
-
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. - PubMed
-
- Krupin T, Cross DA, Becker B. Decreased basal tear production associated with general anesthesia. Arch Ophthalmol. 1977;95:107–108. - PubMed
-
- Semana G, Gausling R, Jackson RA, Hafler DA. T cell autoreactivity to proinsulin epitopes in diabetic patients and healthy subjects. J Autoimmun. 1999;12:259–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

